Long-term Sustainability of Diabetes Prevention Approaches: A Systematic Review and Meta-analysis of Randomized Clinical Trials
- PMID: 29114778
- PMCID: PMC5820728
- DOI: 10.1001/jamainternmed.2017.6040
Long-term Sustainability of Diabetes Prevention Approaches: A Systematic Review and Meta-analysis of Randomized Clinical Trials
Abstract
Importance: Diabetes prevention is imperative to slow worldwide growth of diabetes-related morbidity and mortality. Yet the long-term efficacy of prevention strategies remains unknown.
Objective: To estimate aggregate long-term effects of different diabetes prevention strategies on diabetes incidence.
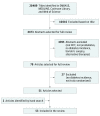
Data sources: Systematic searches of MEDLINE, EMBASE, Cochrane Library, and Web of Science databases. The initial search was conducted on January 14, 2014, and was updated on February 20, 2015. Search terms included prediabetes, primary prevention, and risk reduction.
Study selection: Eligible randomized clinical trials evaluated lifestyle modification (LSM) and medication interventions (>6 months) for diabetes prevention in adults (age ≥18 years) at risk for diabetes, reporting between-group differences in diabetes incidence, published between January 1, 1990, and January 1, 2015. Studies testing alternative therapies and bariatric surgery, as well as those involving participants with gestational diabetes, type 1 or 2 diabetes, and metabolic syndrome, were excluded.
Data extraction and synthesis: Reviewers extracted the number of diabetes cases at the end of active intervention in treatment and control groups. Random-effects meta-analyses were used to obtain pooled relative risks (RRs), and reported incidence rates were used to compute pooled risk differences (RDs).
Main outcomes and measures: The main outcome was aggregate RRs of diabetes in treatment vs control participants. Treatment subtypes (ie, LSM components, medication classes) were stratified. To estimate sustainability, post-washout and follow-up RRs for medications and LSM interventions, respectively, were examined.
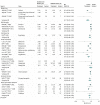
Results: Forty-three studies were included and pooled in meta-analysis (49 029 participants; mean [SD] age, 57.3 [8.7] years; 48.0% [n = 23 549] men): 19 tested medications; 19 evaluated LSM, and 5 tested combined medications and LSM. At the end of the active intervention (range, 0.5-6.3 years), LSM was associated with an RR reduction of 39% (RR, 0.61; 95% CI, 0.54-0.68), and medications were associated with an RR reduction of 36% (RR, 0.64; 95% CI, 0.54-0.76). The observed RD for LSM and medication studies was 4.0 (95% CI, 1.8-6.3) cases per 100 person-years or a number-needed-to-treat of 25. At the end of the washout or follow-up periods, LSM studies (mean follow-up, 7.2 years; range, 5.7-9.4 years) achieved an RR reduction of 28% (RR, 0.72; 95% CI, 0.60-0.86); medication studies (mean follow-up, 17 weeks; range, 2-52 weeks) showed no sustained RR reduction (RR, 0.95; 95% CI, 0.79-1.14).
Conclusions and relevance: In adults at risk for diabetes, LSM and medications (weight loss and insulin-sensitizing agents) successfully reduced diabetes incidence. Medication effects were short lived. The LSM interventions were sustained for several years; however, their effects declined with time, suggesting that interventions to preserve effects are needed.
Conflict of interest statement
Figures

References
-
- International Diabetes Federation Diabetes Atlas—7th Edition www.diabetesatlas.org. Accessed December 15, 2015.
-
- World Health Organization. Global Status Report on Noncommunicable Diseases 2010 Geneva, Switzerland: World Health Organization; 2011.
-
- Stevens JW, Khunti K, Harvey R, et al. . Preventing the progression to type 2 diabetes mellitus in adults at high risk: a systematic review and network meta-analysis of lifestyle, pharmacological and surgical interventions. Diabetes Res Clin Pract. 2015;107(3):320-331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

