Mechanical forces direct stem cell behaviour in development and regeneration
- PMID: 29115301
- PMCID: PMC5803560
- DOI: 10.1038/nrm.2017.108
Mechanical forces direct stem cell behaviour in development and regeneration
Abstract
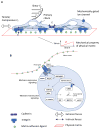
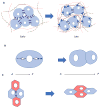
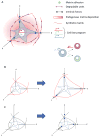
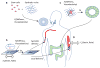
Stem cells and their local microenvironment, or niche, communicate through mechanical cues to regulate cell fate and cell behaviour and to guide developmental processes. During embryonic development, mechanical forces are involved in patterning and organogenesis. The physical environment of pluripotent stem cells regulates their self-renewal and differentiation. Mechanical and physical cues are also important in adult tissues, where adult stem cells require physical interactions with the extracellular matrix to maintain their potency. In vitro, synthetic models of the stem cell niche can be used to precisely control and manipulate the biophysical and biochemical properties of the stem cell microenvironment and to examine how the mode and magnitude of mechanical cues, such as matrix stiffness or applied forces, direct stem cell differentiation and function. Fundamental insights into the mechanobiology of stem cells also inform the design of artificial niches to support stem cells for regenerative therapies.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Steinberg MS. Reconstruction of Tissues by Dissociated Cells. Science. 1963;141:401–408. - PubMed
-
- Ninomiya H, Winklbauer R. Epithelial coating controls mesenchymal shape change through tissue-positioning effects and reduction of surface-minimizing tension. Nature Cell Biology. 2008;10:61–69. - PubMed
-
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. Planar remodeling of cell-cell junctions in embryonic tissue is driven by intrinsic local forces, which are required for germ-band elongation during embryonic development. - PubMed
-
- Beloussov LV, Dorfman JG, Cherdantzev VG. MECHANICAL STRESSES AND MORPHOLOGICAL PATTERNS IN AMPHIBIAN EMBRYOS. Journal of Embryology and Experimental Morphology. 1975;34:559–574. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

