Atorvastatin protects the proliferative ability of human umbilical vein endothelial cells inhibited by angiotensin II by changing mitochondrial energy metabolism
- PMID: 29115384
- PMCID: PMC5746294
- DOI: 10.3892/ijmm.2017.3200
Atorvastatin protects the proliferative ability of human umbilical vein endothelial cells inhibited by angiotensin II by changing mitochondrial energy metabolism
Abstract
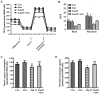
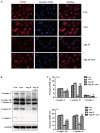
This study aimed to explore whether angiotensin II (Ang II) inhibits the proliferation of human umbilical vein endothelial cells (HUVECs) by changing mitochondrial energy metabolism, and whether atorvastatin has a protective role via restoration of endothelial function. HUVECs were treated with 1 µM Ang II alone or with 10 µM atorvastatin for 24 h. Proliferation was detected by MTT assay, cell counting, 5‑ethynyl‑2'‑deoxyuridine assay and real‑time cell analyzer. Mitochondrial energy metabolism including oxygen consumption rate and extracellular acidification rate were measured using a Seahorse metabolic flux analyzer. Mitochondrial membrane potential was detected under fluorescence microscope following staining with tetramethylrhodamine. Respiratory chain complexes I‑V were detected using western blotting. The current study showed that Ang II inhibits the proliferation of HUVECs. Results from the Seahorse metabolic flux analyzer indicated that Ang II decreased basal oxygen consumption, maximal respiration capacity, spare respiration capacity, adenosine triphosphate‑linked respiration and non‑mitochondrial respiration. By contrast, Ang II increased the proton leak. Additionally, Ang II increased glycolysis, glycolytic capacity and non‑glycolytic acidification. Furthermore, these effects were all suppressed by atorvastatin. The results indicated that atorvastatin prevents cellular energy metabolism switching from oxidative phosphorylation to glycolysis induced by Ang II and protected the proliferative ability of HUVECs.
Figures




Similar articles
-
Atorvastatin inhibits the apoptosis of human umbilical vein endothelial cells induced by angiotensin II via the lysosomal-mitochondrial axis.Apoptosis. 2016 Sep;21(9):977-96. doi: 10.1007/s10495-016-1271-0. Apoptosis. 2016. PMID: 27394920
-
Advanced glycation end products‑induced mitochondrial energy metabolism dysfunction alters proliferation of human umbilical vein endothelial cells.Mol Med Rep. 2017 May;15(5):2673-2680. doi: 10.3892/mmr.2017.6314. Epub 2017 Mar 10. Mol Med Rep. 2017. PMID: 28447727
-
Epigallocatechin-3-Gallate Ameliorates Angiotensin II-Induced Oxidative Stress and Apoptosis in Human Umbilical Vein Endothelial Cells through the Activation of Nrf2/Caspase-3 Signaling.J Vasc Res. 2017;54(5):299-308. doi: 10.1159/000479873. Epub 2017 Sep 23. J Vasc Res. 2017. PMID: 28942440
-
Impact of the renin-angiotensin system on cardiac energy metabolism in heart failure.J Mol Cell Cardiol. 2013 Oct;63:98-106. doi: 10.1016/j.yjmcc.2013.07.010. Epub 2013 Jul 22. J Mol Cell Cardiol. 2013. PMID: 23886814 Review.
-
Zinc inhibition of cellular energy production: implications for mitochondria and neurodegeneration.J Neurochem. 2003 May;85(3):563-70. doi: 10.1046/j.1471-4159.2003.01678.x. J Neurochem. 2003. PMID: 12694382 Review.
Cited by
-
PICK1 modulates glycolysis and angiogenesis of hypoxic endothelial cells by regulating iron homeostasis.Mol Cell Biochem. 2024 May;479(5):1297-1312. doi: 10.1007/s11010-023-04795-z. Epub 2023 Jun 27. Mol Cell Biochem. 2024. PMID: 37368155
-
Evaluation of Glycolysis and Mitochondrial Function in Endothelial Cells Using the Seahorse Analyzer.Methods Mol Biol. 2024;2711:241-256. doi: 10.1007/978-1-0716-3429-5_20. Methods Mol Biol. 2024. PMID: 37776463 Free PMC article.
-
Effects of atorvastatin and ticagrelor combination therapy on renal function and the levels of suppression of tumorigenicity 2 and interleukin-33 in patients with ST-segment elevation myocardial infarction.J Int Med Res. 2020 Dec;48(12):300060520959502. doi: 10.1177/0300060520959502. J Int Med Res. 2020. Retraction in: J Int Med Res. 2024 Jan;52(1):3000605241228165. doi: 10.1177/03000605241228165. PMID: 33275460 Free PMC article. Retracted.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

