Roles and mechanisms of TRPC3 and the PLCγ/PKC/CPI-17 signaling pathway in regulating parturition
- PMID: 29115500
- PMCID: PMC5780171
- DOI: 10.3892/mmr.2017.7998
Roles and mechanisms of TRPC3 and the PLCγ/PKC/CPI-17 signaling pathway in regulating parturition
Erratum in
-
[Corrigendum] Roles and mechanisms of TRPC3 and the PLCγ/PKC/CPI-17 signaling pathway in regulating parturition.Mol Med Rep. 2018 Apr;17(4):6201. doi: 10.3892/mmr.2018.8572. Epub 2018 Feb 7. Mol Med Rep. 2018. PMID: 29436640 Free PMC article.
Abstract
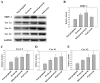
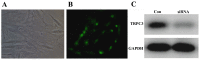
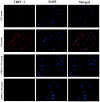
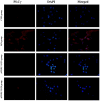
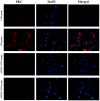
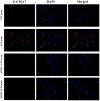
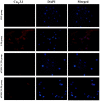
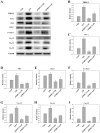
The aim of the current study was to investigate the role of phospholipase C (PLC)γ/protein kinase C (PKC)/C‑kinase‑activated protein phosphatase‑1 (CPI‑17) signaling pathways in uterine smooth muscle during parturition. Samples of uterine tissue were collected from pregnant patients who underwent a caesarean section for preterm delivery, full‑term delivery with labor onset, full‑term delivery without labor onset, and from a non‑pregnant control group undergoing surgery for cervical intraepithelial neoplasia III. Immunohistochemistry, and western blotting were used to assess the association between TRPC3 levels and parturition and the influence of calcium ion channels. In addition, pregnant mice were used to explore the effect of uterine canonical transient receptor potential 3 (TRPC3) expression on the parturition‑triggering mechanism and PLCγ/PKC/CPI‑17 signaling pathways. Pregnant mouse uterine smooth muscle cells were cultivated, with and without TRPC3 silencing, and the expression levels of PLCγ, PKC and CPI‑17, the upstream and downstream factors of the TRPC3 pathway, were measured in pregnant mouse uterine smooth muscle cells, in order to provide a theoretical basis for the prevention and treatment of premature labor. In the preterm and full‑term without labor onset patient groups, the TRPC3 gene expression in the mSMCs was significantly overexpressed when compared with the non‑pregnant group (P<0.05); however, TRPC3 expression was not elevated in the full‑term with labor onset group, exhibiting no significant difference compared with the non‑pregnant group (P>0.05). During pregnancy, compared with the non‑pregnant controls, Cav1.2, Cav3.1 and Cav3.2 gene expression levels were markedly increased (P<0.05) in mSMCs from the preterm delivery group and the full‑term with labor onset group, however were non‑significantly increased in the full‑term without labor onset group. The level of TRPC3 was highest in the preterm group, while the levels of Cav1.2, Cav3.1 and Cav3.2 were highest in the full‑term with labor onset group. In the preterm, LPS‑treated preterm and full‑term groups, TRPC3, MAPK, ERK1/2, P‑ERK, Cav3.2, Cav3.1 and Cav1.2 were all expressed at higher levels than in the unfertilized group. In the LPS‑treated preterm group, the levels of TRPC3, MAPK, ERK1/2, P‑ERK, Cav3.2, Cav3.1 and Cav1.2 were increased compared with the preterm group. Furthermore, following transfection of small interfering TRPC3 (siTRPC3) into cells, it was demonstrated that the levels of TRPC3, PLCγ, PKC, CPI‑17, P‑CPI‑17, Cav1.2, Cav3.1 and Cav3.2 expression were lower in the LPS siTRPC3 group when compared with that of the LPS‑treated untransfected control group.
Figures




References
-
- Senturk MB, Cakmak Y, Gündoğdu M, Polat M, Atac H. Does performing cesarean section after onset of labor has positive effect on neonatal respiratory disorders? J Matern Fetal Neonatal Med. 2016;29:2457–2460. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

