Cost-effectiveness analysis of infant pneumococcal vaccination with PHiD-CV in Korea
- PMID: 29115905
- PMCID: PMC5791581
- DOI: 10.1080/21645515.2017.1362513
Cost-effectiveness analysis of infant pneumococcal vaccination with PHiD-CV in Korea
Abstract
Background: Streptococcus pneumoniae and non-typeable Haemophilus influenzae (NTHi) can cause invasive pneumococcal diseases (IPD), pneumonia, and acute otitis media (AOM). Both the 10-valent pneumococcal NTHi protein D conjugate vaccine (PHiD-CV) and the 13-valent pneumococcal conjugate vaccine (PCV-13) are included in the National Immunization Program for infants in Korea. This study aimed to evaluate the cost-effectiveness of the 3+1 schedule of PHiD-CV versus that of PCV-13 for National Immunization Program in Korea.
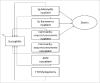
Methods: A published Markov model was adapted to evaluate the cost-effectiveness of vaccinating the 2012 birth cohort with PHiD-CV vs. PCV-13 from the Korean government perspective over 10 y. Best available published data were used for epidemiology, vaccine efficacy and disutilities. Data on incidence and direct medical costs were taken from the national insurance claims database. Sensitivity analyses were conducted to explore the robustness of the results.
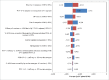
Results: PHiD-CV was projected to prevent an additional 195,262 cases of pneumococcal diseases and NTHi-related diseases vs. PCV-13, with a substantially greater reduction in NTHi-related AOM and a comparable reduction in IPD and community-acquired pneumonia. Parity-priced PHiD-CV generated a health gain of about 844 quality-adjusted life years and a total cost-saving of approximately 4 million United States Dollars (USD) over 10 y. 93% of probabilistic simulations found PHiD-CV 3+1 to be the dominant vaccine option.
Conclusion: Compared to PCV-13, PHiD-CV was projected to provide similar prevention against IPD and community-acquired pneumonia but would prevent more cases of AOM. Parity-priced PHiD-CV was anticipated to generate substantial cost-savings and health benefits vs. PCV-13 in Korea.
Keywords: Korea; PHiD-CV; cost-effectiveness; pneumococcal; vaccine.
Figures
Similar articles
-
Cost-effectiveness analysis of routine pneumococcal vaccination in the UK: a comparison of the PHiD-CV vaccine and the PCV-13 vaccine using a Markov model.BMJ Open. 2016 Nov 30;6(11):e010776. doi: 10.1136/bmjopen-2015-010776. BMJ Open. 2016. PMID: 27903558 Free PMC article.
-
Modeling the impact of a new vaccine on pneumococcal and nontypable Haemophilus influenzae diseases: a new simulation model.Clin Ther. 2009 Oct;31(10):2152-69. doi: 10.1016/j.clinthera.2009.10.014. Clin Ther. 2009. PMID: 19922887
-
Overall effectiveness of pneumococcal conjugate vaccines: An economic analysis of PHiD-CV and PCV-13 in the immunization of infants in Italy.Hum Vaccin Immunother. 2017 Oct 3;13(10):2307-2315. doi: 10.1080/21645515.2017.1343773. Epub 2017 Jul 12. Hum Vaccin Immunother. 2017. PMID: 28700264 Free PMC article.
-
Serotype distribution of invasive and non-invasive pneumococcal disease in children ≤5 years of age following the introduction of 10- and 13-valent pneumococcal conjugate vaccines in infant national immunization programs: a systematic literature review.Front Public Health. 2025 May 30;13:1544359. doi: 10.3389/fpubh.2025.1544359. eCollection 2025. Front Public Health. 2025. PMID: 40520299 Free PMC article.
-
Impact of protein D-containing pneumococcal conjugate vaccines on non-typeable Haemophilus influenzae acute otitis media and carriage.Expert Rev Vaccines. 2017 Jul;16(7):1-14. doi: 10.1080/14760584.2017.1333905. Epub 2017 Jun 7. Expert Rev Vaccines. 2017. PMID: 28571504 Review.
Cited by
-
Pneumococcal Vaccination for Children in Asian Countries: A Systematic Review of Economic Evaluation Studies.Vaccines (Basel). 2020 Jul 30;8(3):426. doi: 10.3390/vaccines8030426. Vaccines (Basel). 2020. PMID: 32751569 Free PMC article. Review.
-
Response to McGirr et al.'s Comment on "Clinical and Economic Impact of a Potential Switch from 13-Valent to 10-Valent Pneumococcal Conjugate Infant Vaccination in Canada".Infect Dis Ther. 2018 Dec;7(4):539-543. doi: 10.1007/s40121-018-0221-2. Epub 2018 Nov 8. Infect Dis Ther. 2018. PMID: 30411203 Free PMC article. No abstract available.
-
Cost-Effectiveness and Budget Impact Analyses of Pneumococcal Vaccination in Indonesia.J Environ Public Health. 2021 Apr 27;2021:7494965. doi: 10.1155/2021/7494965. eCollection 2021. J Environ Public Health. 2021. PMID: 33995536 Free PMC article.
-
Appropriateness of strategy comparisons in cost-effectiveness analyses of infant pneumococcal vaccination: a systematic review.Int J Technol Assess Health Care. 2023 Jul 12;39(1):e42. doi: 10.1017/S0266462323000351. Int J Technol Assess Health Care. 2023. PMID: 37435736 Free PMC article.
-
Health and economic burden associated with 15-valent pneumococcal conjugate vaccine serotypes in Korea and Hong Kong.Hum Vaccin Immunother. 2022 Nov 30;18(5):2046433. doi: 10.1080/21645515.2022.2046433. Epub 2022 Apr 14. Hum Vaccin Immunother. 2022. PMID: 35420975 Free PMC article.
References
-
- Center for Disease Control and Prevention (CDC ). Prevention of Pneumococcal Disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations Reports. 1997;46(RR-08):1-24. - PubMed
-
- O'Brien KL, Shaw J, Weatherholtz R, Reid R, Watt J, Croll J, Dagan R, Parkinson AJ, Santosham M. Epidemiology of invasive Streptococcus pneumoniae among Navajo children in the era before use of conjugate pneumococcal vaccines, 1989-1996. Am J Epidemiol. 2004;160(3):270-8. doi:10.1093/aje/kwh191. PMID:15258000 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
