Trastuzumab resumption after extremely severe cardiotoxicity in metastatic breast cancer patient: a case report
- PMID: 29115937
- PMCID: PMC5678795
- DOI: 10.1186/s12885-017-3712-8
Trastuzumab resumption after extremely severe cardiotoxicity in metastatic breast cancer patient: a case report
Abstract
Background: Trastuzumab-related cardiotoxicity has been reported in patients receiving trastuzumab concurrently with other agents, especially with anthracyclines. Cardiac function damage is generally rare, precox and mild with trastuzumab alone.
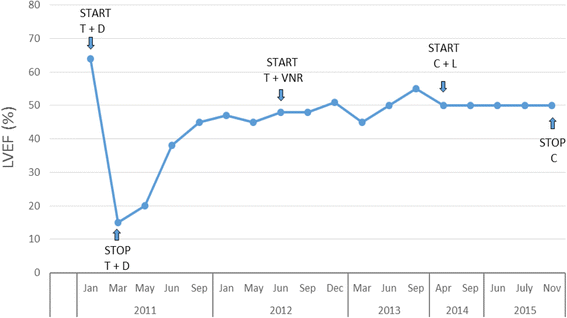
Case presentation: We report the case of a 49 year-old woman affected by metastatic breast cancer who developed trastuzumab-related cardiogenic shock due to pump failure (with LVEF of about 15%) after three months of treatment. After a long hospitalization in the cardiac intensive care unit and a proper treatment, LVEF increased to 50% and, due to a severe progression of disease, trastuzumab was resumed and continued for more than one year.
Conclusion: This is a case of particularly severe cardiotoxicity related to trastuzumab treatment, which was recovered with pharmacological treatment and the temporary discontinuation of the treatment. Trastuzumab was safely resumed after clinical and echocardiographic parameters improvement.
Keywords: Breast cancer; Cardiotoxicity; Ejection fraction; Heart failure; Monoclonal antibody; Trastuzumab.
Conflict of interest statement
Ethics approval and consent to participate
The authors declare they have observed appropriate ethical guidelines and legislation in writing the case report. Consent to participate was obtained from the patient.
Consent for publication
Written informed consent was obtained from the patient for publication of this Case Report. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Keefe Deborah L. Trastuzumab-associated cardiotoxicity. CANCER October 1, 2002 / Volume 95 / Number 7. - PubMed
-
- Martín M, Esteva FJ, Alba E, Khandheria B, Pérez-Isla L, García-Sáenz JA, Márquez A, Sengupta P, Zamorano J. Minimizing cardiotoxicity while optimizing treatment efficacy with trastuzumab: review and expert recommendations. Oncologist. 2009 Jan;14(1):1–11. doi: 10.1634/theoncologist.2008-0137. Epub 2009 Jan 15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical