Changes in vasoactive pathways in congenital diaphragmatic hernia associated pulmonary hypertension explain unresponsiveness to pharmacotherapy
- PMID: 29115963
- PMCID: PMC5688796
- DOI: 10.1186/s12931-017-0670-2
Changes in vasoactive pathways in congenital diaphragmatic hernia associated pulmonary hypertension explain unresponsiveness to pharmacotherapy
Abstract
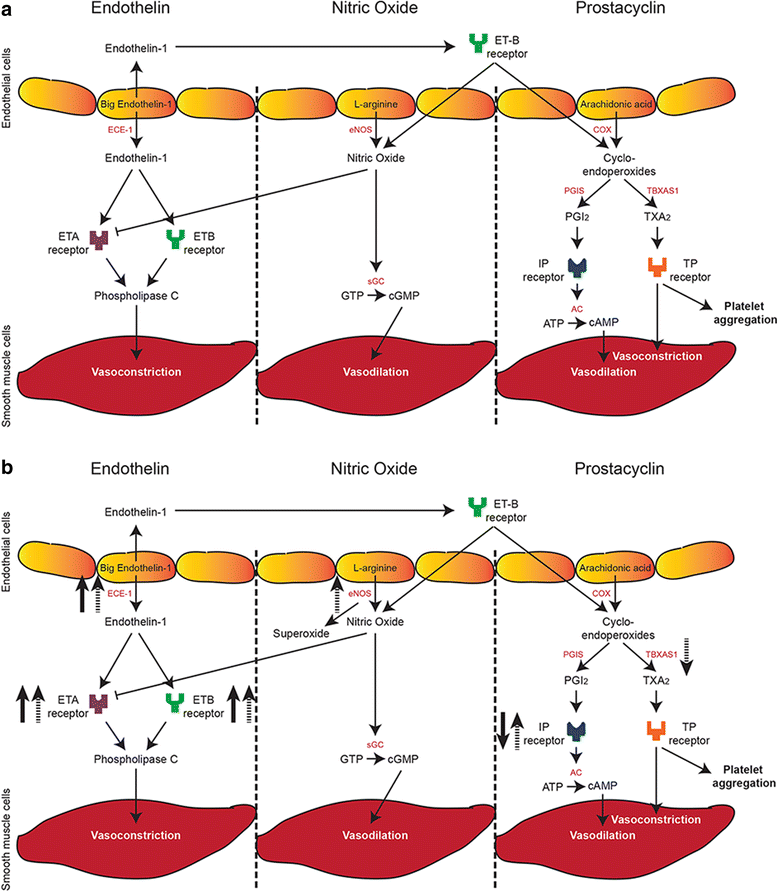
Background: Patients with congenital diaphragmatic hernia (CDH) have structural and functional different pulmonary vessels, leading to pulmonary hypertension. They often fail to respond to standard vasodilator therapy targeting the major vasoactive pathways, causing a high morbidity and mortality. We analyzed whether the expression of crucial members of these vasoactive pathways could explain the lack of responsiveness to therapy in CDH patients.
Methods: The expression of direct targets of current vasodilator therapy in the endothelin and prostacyclin pathway was analyzed in human lung specimens of control and CDH patients.
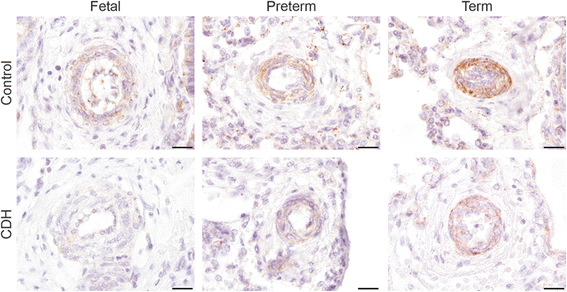
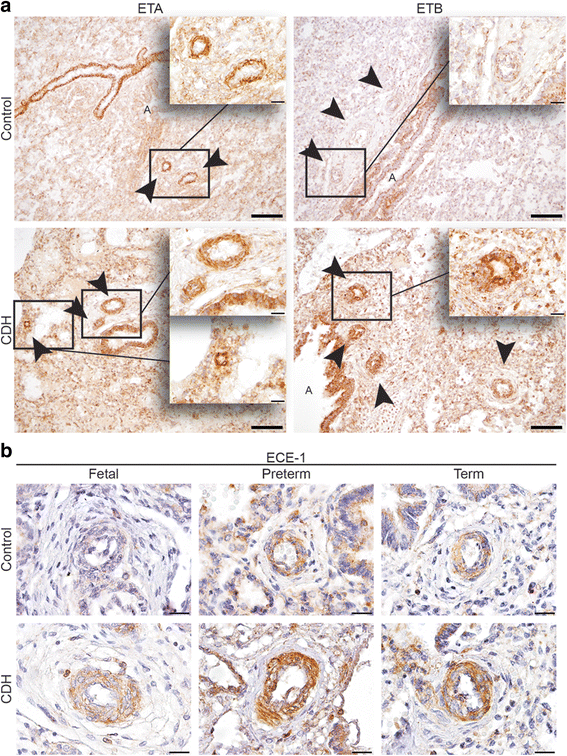
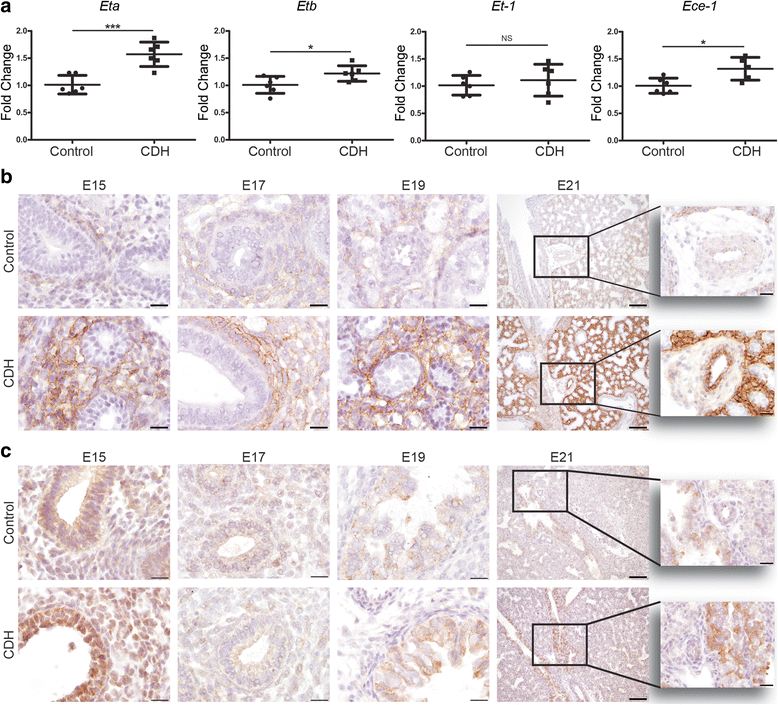
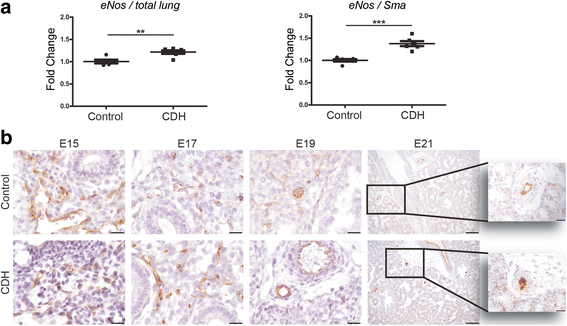
Results: CDH lungs showed increased expression of both ETA and ETB endothelin receptors and the rate-limiting Endothelin Converting Enzyme (ECE-1), and a decreased expression of the prostaglandin-I2 receptor (PTGIR). These data were supported by increased expression of both endothelin receptors and ECE-1, endothelial nitric oxide synthase and PTGIR in the well-established nitrofen-CDH rodent model.
Conclusions: Together, these data demonstrate aberrant expression of targeted receptors in the endothelin and prostacyclin pathway in CDH already early during development. The analysis of this unique patient material may explain why a significant number of patients do not respond to vasodilator therapy. This knowledge could have important implications for the choice of drugs and the design of future clinical trials internationally.
Keywords: Development; Endothelin; Lung; Nitric oxide; Prostacyclin; Vasculature; Vasodilation.
Conflict of interest statement
Ethics approval and consent to participate
Human lung samples were retrieved from the archives of the Department of Pathology of the Erasmus Medical Center, Rotterdam, following approval by the Erasmus MC Medical Ethical Committee. According to Dutch law following consent to perform autopsy, no separate consent is needed from parents to perform additional staining of tissues.
All animal experiments were approved by an independent animal ethical committee and according to national guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Perturbations in Endothelial Dysfunction-Associated Pathways in the Nitrofen-Induced Congenital Diaphragmatic Hernia Model.J Vasc Res. 2018;55(1):26-34. doi: 10.1159/000484087. Epub 2017 Dec 8. J Vasc Res. 2018. PMID: 29216632
-
Pulmonary vascular balance in congenital diaphragmatic hernia: enhanced endothelin-1 gene expression as a possible cause of pulmonary vasoconstriction.J Pediatr Surg. 1998 Jan;33(1):81-4. doi: 10.1016/s0022-3468(98)90367-0. J Pediatr Surg. 1998. PMID: 9473106
-
Endothelin-1 pulmonary vasoconstriction in rats with diaphragmatic hernia.J Surg Res. 1998 Apr;76(1):74-8. doi: 10.1006/jsre.1997.5293. J Surg Res. 1998. PMID: 9695743
-
The Use of Inhaled Nitric Oxide in Congenital Diaphragmatic Hernia.Adv Neonatal Care. 2020 Dec;20(6):479-486. doi: 10.1097/ANC.0000000000000753. Adv Neonatal Care. 2020. PMID: 32384329 Review.
-
Congenital diaphragmatic hernia-associated pulmonary hypertension.Semin Perinatol. 2020 Feb;44(1):151167. doi: 10.1053/j.semperi.2019.07.006. Epub 2019 Jul 30. Semin Perinatol. 2020. PMID: 31519366 Review.
Cited by
-
A proteome signature of umbilical cord serum associated with congenital diaphragmatic hernia.Nagoya J Med Sci. 2020 May;82(2):345-354. doi: 10.18999/nagjms.82.2.345. Nagoya J Med Sci. 2020. PMID: 32581413 Free PMC article.
-
Inhaled Nitric Oxide in Neonatal Pulmonary Hypertension.Clin Perinatol. 2024 Mar;51(1):95-111. doi: 10.1016/j.clp.2023.11.001. Epub 2023 Nov 29. Clin Perinatol. 2024. PMID: 38325949 Free PMC article. Review.
-
Challenges and Pitfalls: Performing Clinical Trials in Patients With Congenital Diaphragmatic Hernia.Front Pediatr. 2022 Apr 15;10:852843. doi: 10.3389/fped.2022.852843. eCollection 2022. Front Pediatr. 2022. PMID: 35498783 Free PMC article. Review.
-
Impact of Fgf10 deficiency on pulmonary vasculature formation in a mouse model of bronchopulmonary dysplasia.Hum Mol Genet. 2019 May 1;28(9):1429-1444. doi: 10.1093/hmg/ddy439. Hum Mol Genet. 2019. PMID: 30566624 Free PMC article.
-
Congenital diaphragmatic hernia: a narrative review of controversies in neonatal management.Transl Pediatr. 2021 May;10(5):1432-1447. doi: 10.21037/tp-20-142. Transl Pediatr. 2021. PMID: 34189103 Free PMC article. Review.
References
-
- Puligandla PS, Grabowski J, Austin M, Hedrick H, Renaud E, Arnold M, Williams RF, Graziano K, Dasgupta R, McKee M, et al. Management of congenital diaphragmatic hernia: a systematic review from the APSA outcomes and evidence based practice committee. J Pediatr Surg. 2015;50:1958–1970. doi: 10.1016/j.jpedsurg.2015.09.010. - DOI - PubMed
-
- Snoek KG, Reiss IK, Greenough A, Capolupo I, Urlesberger B, Wessel L, Storme L, Deprest J, Schaible T, van Heijst A, et al. Standardized Postnatal Management of Infants with Congenital Diaphragmatic Hernia in Europe: The CDH EURO Consortium Consensus - 2015 Update. Neonatology. 2016;110:66–74. doi: 10.1159/000444210. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

