STAT3-blocked whole-cell hepatoma vaccine induces cellular and humoral immune response against HCC
- PMID: 29115974
- PMCID: PMC5688805
- DOI: 10.1186/s13046-017-0623-0
STAT3-blocked whole-cell hepatoma vaccine induces cellular and humoral immune response against HCC
Abstract
Background: Whole-cell tumor vaccines have shown much promise; however, only limited success has been achieved for the goal of eliciting robust tumor-specific T-cell responses.
Methods: Hepatocellular carcinoma (HCC) cells, H22 and Hepa1-6, were modified by blocking the STAT3 signaling pathway with a STAT3 decoy oligodeoxynucleotide, and the immunogenicity and possibility of using these cell lysates as a vaccine were evaluated.
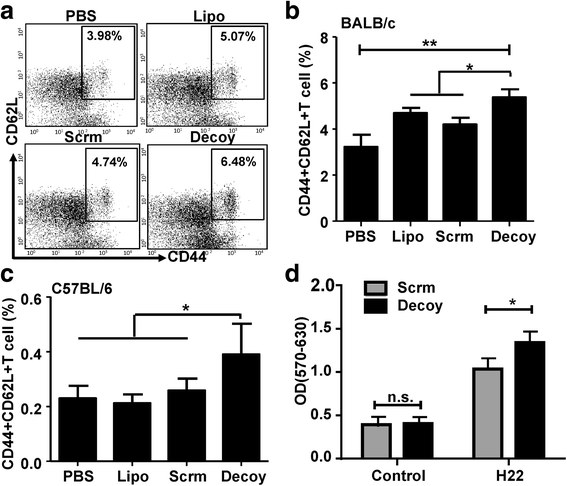
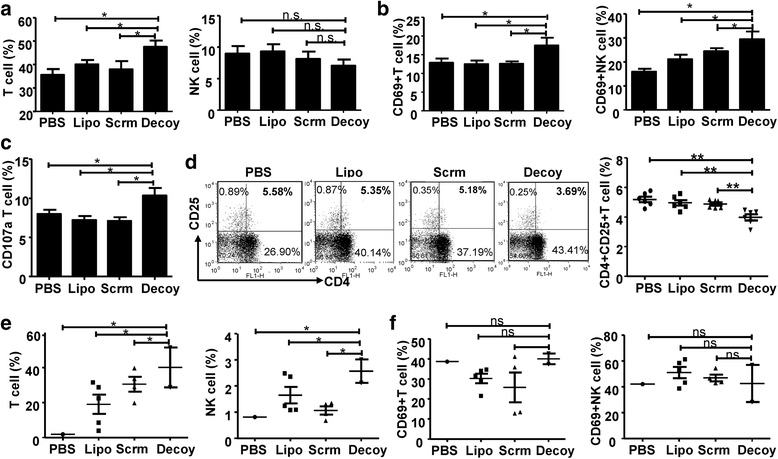
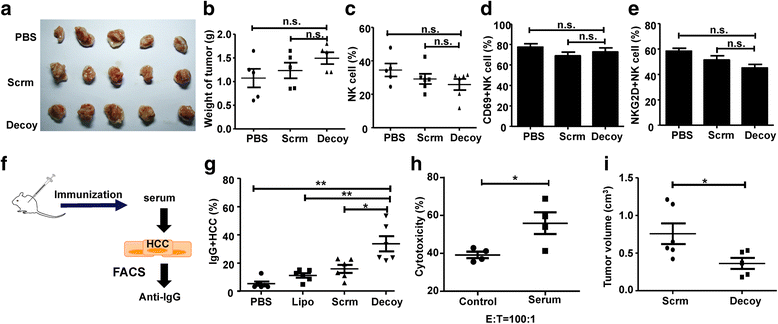
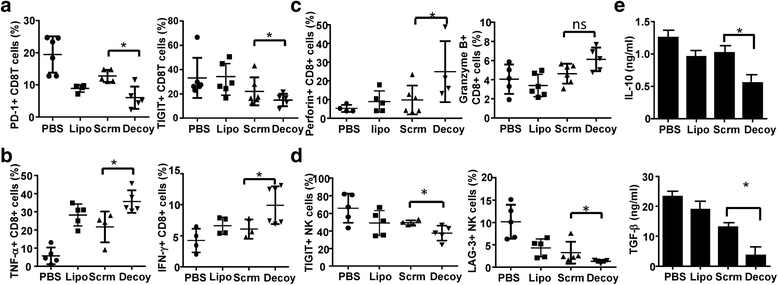
Results: STAT3-blocked whole HCC cell lysates inhibited tumor growth and tumorigenesis, and prolonged the survival of tumor-bearing mice. In addition, STAT3-blocked whole HCC cell lysates stimulated the activation of T cells and natural killer (NK) cells, and enhanced the infiltration of cytotoxic CD8+ T cells in the tumor tissues. In addition, the maturation of dendritic cells (DCs) was enhanced, which promoted the generation of immunological memory against HCC. Furthermore, secondary immune responses could be primed as soon as these immunized mice were challenged with HCC cells, accompanied by T cell and NK cell activation and infiltration. Additionally, immunization with this vaccine decreased the generation of Tregs and the production of TGF-β and IL-10. Importantly, STAT3-blocked whole HCC cell lysates prevented HCC-mediated exhaustion of T cells and NK cells, showing low expression of checkpoint molecules such as PD-1 and TIGIT on T cells and NK cells in the immunized mice.
Conclusions: The newly generated STAT3-blocked whole-cell HCC vaccine has potential for cancer cell vaccination.
Keywords: Hepatoma; Immunotherapy; STAT3; Tumor vaccine; Whole-cell vaccine.
Conflict of interest statement
Ethics approval and consent to participate
The animal research was approved by the Animal Research Ethics Committee of Shandong University, and complied with the Guidelines for Animal Experiments of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Greten TF, Forner A, Korangy F, N'Kontchou G, Barget N, Ayuso C, Ormandy LA, Manns MP, Beaugrand M, Bruix J. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer. 2010;10:209. doi: 10.1186/1471-2407-10-209. - DOI - PMC - PubMed
-
- Saini N, Srinivasan R, Chawla Y, Sharma S, Chakraborti A, Rajwanshi A. Telomerase activity, telomere length and human telomerase reverse transcriptase expression in hepatocellular carcinoma is independent of hepatitis virus status. Liver Int. 2009;29(8):1162–1170. doi: 10.1111/j.1478-3231.2009.02082.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

