Composite intestinal adenoma-microcarcinoid in the colon and rectum: a case series and historical review
- PMID: 29116005
- PMCID: PMC5688820
- DOI: 10.1186/s13000-017-0665-9
Composite intestinal adenoma-microcarcinoid in the colon and rectum: a case series and historical review
Abstract
Background: Composite intestinal adenoma-microcarcinoid (CIAM) is a rare colorectal lesion that mostly comprises a conventional adenomatous component with a minute proportion of neuroendocrine (NE) component. Although microcarcinoids are well-recognized in the setting of chronic inflammatory disorders of the gastrointestinal tract, large intestinal microcarcinoids associated with intestinal adenoma are exceedingly rare and their clinicopathologic characteristics are yet to be elucidated. This study was performed to clarify their clinicopathologic characteristics and to review the relevant literature.
Methods: In total, 24 cases of CIAM in which tumors were excised endoscopically (n = 22) or surgically (n = 2) were retrieved from the Department of Pathology, Daehang Hospital. We analyzed their clinicopathologic characteristics and performed immunohistochemical staining for NE markers to determine their endocrine nature.
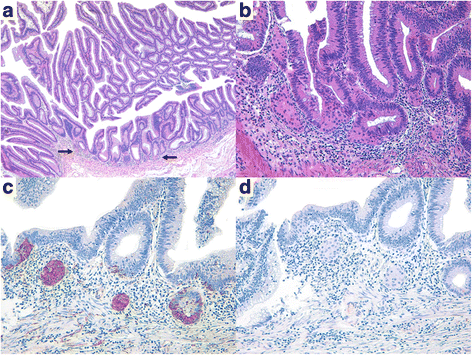
Results: CIAM usually developed in middle-aged and elderly patients, with a mean age of 62.0 years (range, 44-81 years). Thirteen patients were men and 11 were women, indicating a nearly equal sex ratio. Unlike classic carcinoid tumors, CIAMs occurred mostly in the colon (83.3% of cases), particularly in the proximal colon. Histologically, the microcarcinoid component consisted of low-grade NE cells arranged in small nests, glands or cords interspersed with glandular elements or less frequently resembled squamous morules. There was no expansile nodular or organoid growth pattern, which is typical of carcinoid tumors. The microcarcinoids were 1-20 mm in size (mean size, 4.7 mm) and were mostly situated in the basal lamina propria with no submucosal layer involvement; none showed desmoplastic reaction or increased proliferative activity. Follow-up data (mean, 23.1 months) were available for 18 patients; all patients are alive and well.
Conclusions: To the best of our knowledge, ours is the largest series of patients with CIAM in the English-language literature. Microcarcinoids found in CIAMs appear to show favorable clinical outcomes regardless of their size, likely due to the absence of submucosal extension and/or increased proliferative activity. We recommend avoiding additional radical surgeries in patients who have endoscopically undergone complete CIAM excision unless they exhibit ominous histologic features such as submucosal extension or increased proliferative activity.
Keywords: Colorectal lesions; Composite intestinal adenoma; Microcarcinoid; Neuroendocrine tumor.
Conflict of interest statement
Ethics approval and consent to participate
The study was performed according to the Declaration of Helsinki, and was approved by the institutional review board at Daehang Hospital (approval number DH17–001). Obtaining additional informed consent for the use of patient samples was not required, as approved by the institutional review board. Specimens were coded to protect patient confidentiality.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Reinecke P, Borchard F. Pattern of gastric endocrine cells in microcarcinoidosis--an immunohistochemical study of 14 gastric biopsies. Virchows Arch. 1996;428:237–241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

