Single cell transcriptomics suggest that human adipocyte progenitor cells constitute a homogeneous cell population
- PMID: 29116032
- PMCID: PMC5678572
- DOI: 10.1186/s13287-017-0701-4
Single cell transcriptomics suggest that human adipocyte progenitor cells constitute a homogeneous cell population
Abstract
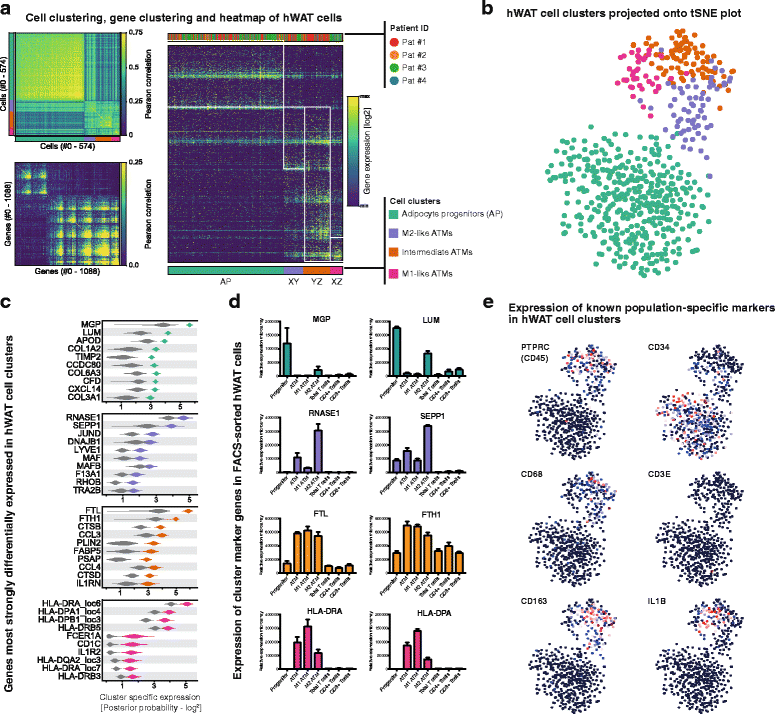
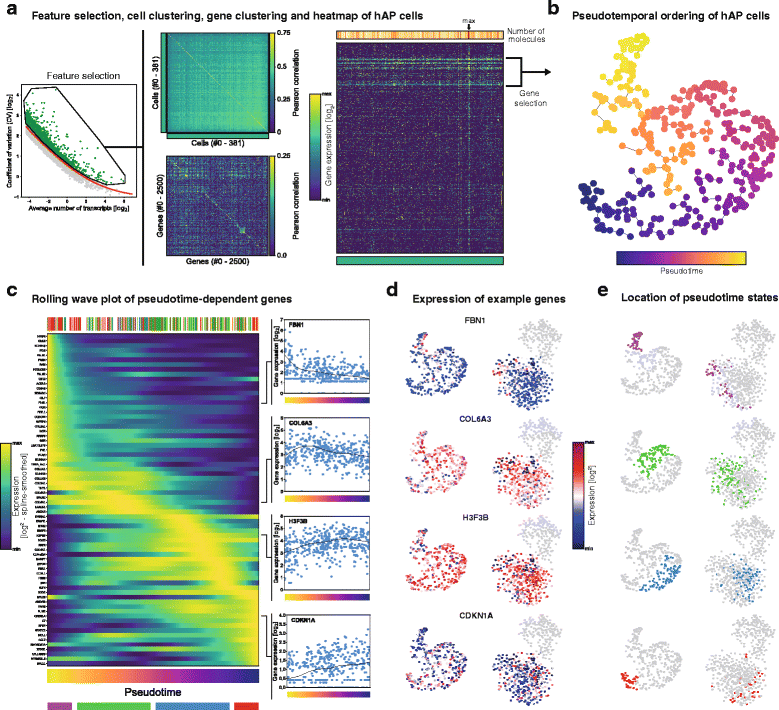
Regulation of adipose tissue stem cells (ASCs) and adipogenesis impact the development of excess body fat-related metabolic complications. Animal studies have suggested the presence of distinct subtypes of ASCs with different differentiation properties. In addition, ASCs are becoming the biggest source of mesenchymal stem cells used in therapies, which requires deep characterization. Using unbiased single cell transcriptomics we aimed to characterize ASC populations in human subcutaneous white adipose tissue (scWAT). The transcriptomes of 574 single cells from the WAT total stroma vascular fraction (SVF) of four healthy women were analyzed by clustering and t-distributed stochastic neighbor embedding visualization. The identified cell populations were then mapped to cell types present in WAT using data from gene expression microarray profiling of flow cytometry-sorted SVF. Cells clustered into four distinct populations: three adipose tissue-resident macrophage subtypes and one large, homogeneous population of ASCs. While pseudotemporal ordering analysis indicated that the ASCs were in slightly different differentiation stages, the differences in gene expression were small and could not distinguish distinct ASC subtypes. Altogether, in healthy individuals, ASCs seem to constitute a single homogeneous cell population that cannot be subdivided by single cell transcriptomics, suggesting a common origin for human adipocytes in scWAT.
Keywords: Adipocyte progenitor; Human adipose tissue; Mesenchymal stem cells; Single cell sequencing.
Conflict of interest statement
Authors’ information
KK’s current address is Departments of Medicine and Genetics, Stanford University, Stanford, CA 94305, USA.
Ethics approval and consent to participate
The study was approved by the regional ethics board at Karolinska Institutet and all subjects provided their written informed consent.
Consent for publication
Written informed consent was obtained from the patients for recording/using their individual details (age, gender, weight, and height) for research purposes. Samples were coded at the collection point and cannot by any means be connected to personal identity. The consent form is held in the patients’ clinical notes and is available for review by the Editor-in-Chief.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

