Preservation of Functional Microvascular Bed Is Vital for Long-Term Survival of Cardiac Myocytes Within Large Transmural Post-Myocardial Infarction Scar
- PMID: 29116876
- PMCID: PMC5794202
- DOI: 10.1369/0022155417741640
Preservation of Functional Microvascular Bed Is Vital for Long-Term Survival of Cardiac Myocytes Within Large Transmural Post-Myocardial Infarction Scar
Abstract
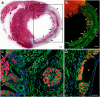
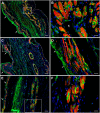
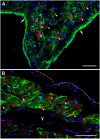
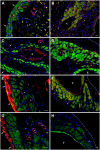
This study was aimed to understand the mechanism of persistent cardiac myocyte (CM) survival in myocardial infarction (MI) scars. A transmural MI was induced in 12-month-old Sprague-Dawley rats by permanent coronary artery ligation. The hearts were collected 3 days, 1, 2, 4, 8, and 12 weeks after MI and evaluated with histology, immunohistochemistry, and quantitative morphometry. Vasculature patency was assessed in 4-, 8-, and 12-week-old scars by infusion of 15-micron microspheres into the left ventricle before euthanasia. The infarcted/scarred area has a small continually retained population of surviving CMs in subendocardial and subepicardial regions. Surprisingly, whereas the transverse area of subepicardial CMs remained relatively preserved or even enlarged over 12 post-MI weeks, subendocardial CMs underwent progressive atrophy. Nevertheless, the fractional volume of viable CMs remained comparable in mature scars 4, 8, and 12 weeks after MI (3.6 ± 0.4%, 3.4 ± 0.5%, and 2.5 ± 0.3%, respectively). Despite the opposite dynamics of changes in size, CMs of both regions displayed sarcomeres and gap junctions. Most importantly, surviving CMs were always accompanied by patent microvessels linked to a venous network composed of Thebesian veins, intramural sinusoids, and subepicardial veins. Our findings reveal that long-term survival of CMs in transmural post-MI scars is sustained by a local microcirculatory bed.
Keywords: Thebesian vessels; cardiac myocyte survival; microvascular beds; middle-aged rats; myocardial infarction; scar formation; subepicardial veins; venous sinusoids.
Conflict of interest statement
Figures








Similar articles
-
Large- and Medium-sized Arteries Remaining in Transmural Scar Distal to Permanent Coronary Ligation Undergo Neointimal Hyperplasia and Inward Remodeling.J Histochem Cytochem. 2021 May;69(5):321-338. doi: 10.1369/00221554211004297. Epub 2021 Mar 22. J Histochem Cytochem. 2021. PMID: 33749360 Free PMC article.
-
Structural composition of myocardial infarction scar in middle-aged male and female rats: does sex matter?J Histochem Cytochem. 2013 Nov;61(11):833-48. doi: 10.1369/0022155413499794. Epub 2013 Jul 18. J Histochem Cytochem. 2013. PMID: 23867842 Free PMC article.
-
Cardiomyocytes from CCND2-overexpressing human induced-pluripotent stem cells repopulate the myocardial scar in mice: A 6-month study.J Mol Cell Cardiol. 2019 Dec;137:25-33. doi: 10.1016/j.yjmcc.2019.09.011. Epub 2019 Oct 17. J Mol Cell Cardiol. 2019. PMID: 31629738 Free PMC article.
-
Biomechanical properties of acellular scar ECM during the acute to chronic stages of myocardial infarction.J Mech Behav Biomed Mater. 2021 Apr;116:104342. doi: 10.1016/j.jmbbm.2021.104342. Epub 2021 Jan 22. J Mech Behav Biomed Mater. 2021. PMID: 33516128 Free PMC article.
-
Expression of Gq alpha and PLC-beta in scar and border tissue in heart failure due to myocardial infarction.Circulation. 1998 Mar 10;97(9):892-9. doi: 10.1161/01.cir.97.9.892. Circulation. 1998. PMID: 9521338
Cited by
-
Rebuilding the myocardial microenvironment to enhance mesenchymal stem cells-mediated regeneration in ischemic heart disease.Front Bioeng Biotechnol. 2024 Sep 20;12:1468833. doi: 10.3389/fbioe.2024.1468833. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39372432 Free PMC article. Review.
-
Extracellular matrix remodeling is associated with the survival of cardiomyocytes in the subendocardial region of the ischemic myocardium.Exp Biol Med (Maywood). 2021 Dec;246(24):2579-2588. doi: 10.1177/15353702211042020. Epub 2021 Sep 13. Exp Biol Med (Maywood). 2021. PMID: 34515546 Free PMC article.
-
Large- and Medium-sized Arteries Remaining in Transmural Scar Distal to Permanent Coronary Ligation Undergo Neointimal Hyperplasia and Inward Remodeling.J Histochem Cytochem. 2021 May;69(5):321-338. doi: 10.1369/00221554211004297. Epub 2021 Mar 22. J Histochem Cytochem. 2021. PMID: 33749360 Free PMC article.
-
Engineering the multiscale complexity of vascular networks.Nat Rev Mater. 2022;7(9):702-716. doi: 10.1038/s41578-022-00447-8. Epub 2022 May 31. Nat Rev Mater. 2022. PMID: 35669037 Free PMC article. Review.
-
Alteration of endothelial permeability ensures cardiomyocyte survival from ischemic insult in the subendocardium of the heart.Exp Biol Med (Maywood). 2023 Aug;248(16):1364-1372. doi: 10.1177/15353702231194344. Epub 2023 Oct 3. Exp Biol Med (Maywood). 2023. PMID: 37786370 Free PMC article.
References
-
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81(4):1161–72. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

