20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years
- PMID: 29117498
- PMCID: PMC5734609
- DOI: 10.1056/NEJMoa1701830
20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years
Abstract
Background: The administration of endocrine therapy for 5 years substantially reduces recurrence rates during and after treatment in women with early-stage, estrogen-receptor (ER)-positive breast cancer. Extending such therapy beyond 5 years offers further protection but has additional side effects. Obtaining data on the absolute risk of subsequent distant recurrence if therapy stops at 5 years could help determine whether to extend treatment.
Methods: In this meta-analysis of the results of 88 trials involving 62,923 women with ER-positive breast cancer who were disease-free after 5 years of scheduled endocrine therapy, we used Kaplan-Meier and Cox regression analyses, stratified according to trial and treatment, to assess the associations of tumor diameter and nodal status (TN), tumor grade, and other factors with patients' outcomes during the period from 5 to 20 years.
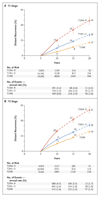
Results: Breast-cancer recurrences occurred at a steady rate throughout the study period from 5 to 20 years. The risk of distant recurrence was strongly correlated with the original TN status. Among the patients with stage T1 disease, the risk of distant recurrence was 13% with no nodal involvement (T1N0), 20% with one to three nodes involved (T1N1-3), and 34% with four to nine nodes involved (T1N4-9); among those with stage T2 disease, the risks were 19% with T2N0, 26% with T2N1-3, and 41% with T2N4-9. The risk of death from breast cancer was similarly dependent on TN status, but the risk of contralateral breast cancer was not. Given the TN status, the factors of tumor grade (available in 43,590 patients) and Ki-67 status (available in 7692 patients), which are strongly correlated with each other, were of only moderate independent predictive value for distant recurrence, but the status regarding the progesterone receptor (in 54,115 patients) and human epidermal growth factor receptor type 2 (HER2) (in 15,418 patients in trials with no use of trastuzumab) was not predictive. During the study period from 5 to 20 years, the absolute risk of distant recurrence among patients with T1N0 breast cancer was 10% for low-grade disease, 13% for moderate-grade disease, and 17% for high-grade disease; the corresponding risks of any recurrence or a contralateral breast cancer were 17%, 22%, and 26%, respectively.
Conclusions: After 5 years of adjuvant endocrine therapy, breast-cancer recurrences continued to occur steadily throughout the study period from 5 to 20 years. The risk of distant recurrence was strongly correlated with the original TN status, with risks ranging from 10 to 41%, depending on TN status and tumor grade. (Funded by Cancer Research UK and others.).
Figures


Comment in
-
A rude awakening from tumour cells.Nature. 2018 Feb 1;554(7690):35-36. doi: 10.1038/d41586-018-01140-z. Nature. 2018. PMID: 29388969 No abstract available.
-
Breast-Cancer Recurrence after Stopping Endocrine Therapy.N Engl J Med. 2018 Mar 1;378(9):870. doi: 10.1056/NEJMc1715968. N Engl J Med. 2018. PMID: 29504722 No abstract available.
-
Progress in breast cancer management.Lancet. 2024 Oct 12;404(10461):1376-1378. doi: 10.1016/S0140-6736(24)01823-3. Lancet. 2024. PMID: 39396338 No abstract available.
References
-
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. - PubMed
-
- The Early Breast Cancer Trialists’ Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–52. - PubMed
-
- Gray RG, Rea DW, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early-stage breast cancer. J Clin Oncol. 2013;31(suppl 5) abstract.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
