CDK8 Kinase Activity Promotes Glycolysis
- PMID: 29117556
- PMCID: PMC5726794
- DOI: 10.1016/j.celrep.2017.10.058
CDK8 Kinase Activity Promotes Glycolysis
Abstract
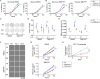
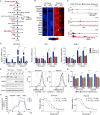
Aerobic glycolysis, also known as the Warburg effect, is a hallmark of cancerous tissues. Despite its importance in cancer development, our understanding of mechanisms driving this form of metabolic reprogramming is incomplete. We report here an analysis of colorectal cancer cells engineered to carry a single point mutation in the active site of the Mediator-associated kinase CDK8, creating hypomorphic alleles sensitive to bulky ATP analogs. Transcriptome analysis revealed that CDK8 kinase activity is required for the expression of many components of the glycolytic cascade. CDK8 inhibition impairs glucose transporter expression, glucose uptake, glycolytic capacity and reserve, as well as cell proliferation and anchorage-independent growth, both in normoxia and hypoxia. Importantly, CDK8 impairment sensitizes cells to pharmacological glycolysis inhibition, a result reproduced with Senexin A, a dual inhibitor of CDK8/CDK19. Altogether, these results contribute to our understanding of CDK8 as an oncogene, and they justify investigations to target CDK8 in highly glycolytic tumors.
Keywords: A549; CDK19; CDK8; H460; HCT116; Mediator; SW480; Warburg effect; chemical genetics; glycolysis.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
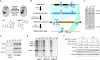


Comment in
-
Taking Aim at Glycolysis with CDK8 Inhibitors.Trends Endocrinol Metab. 2018 May;29(5):281-282. doi: 10.1016/j.tem.2018.02.005. Epub 2018 Feb 20. Trends Endocrinol Metab. 2018. PMID: 29475579 Free PMC article.
References
-
- Barthel A, Okino ST, Liao J, Nakatani K, Li J, Whitlock JP, Jr, Roth RA. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J Biol Chem. 1999;274:20281–20286. - PubMed
-
- Blethrow J, Zhang C, Shokat KM, Weiss EL. Design and use of analog-sensitive protein kinases. Curr Protoc Mol Biol Chapter. 2004;18 Unit 18 11. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

