Medical and dietary interventions for preventing recurrent urinary stones in children
- PMID: 29117629
- PMCID: PMC6486163
- DOI: 10.1002/14651858.CD011252.pub2
Medical and dietary interventions for preventing recurrent urinary stones in children
Abstract
Background: Nephrolithiasis, or urinary stone disease, in children causes significant morbidity, and is increasing in prevalence in the North American population. Therefore, medical and dietary interventions (MDI) for recurrent urinary stones in children are poised to gain increasing importance in the clinical armamentarium.
Objectives: To assess the effects of medical and dietary interventions (MDI) for the prevention of idiopathic urinary stones in children aged from one to 18 years.
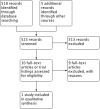
Search methods: We searched multiple databases using search terms relevant to this review, including studies identified from the Cochrane Central Register of Controlled Trials (CENTRAL, 2017, Issue 1), MEDLINE OvidSP (1946 to 14 February 2017), Embase OvidSP (1980 to 14 February 2017), International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov. Additionally, we handsearched renal-related journals and the proceedings of major renal conferences, and reviewed weekly current awareness alerts for selected renal journals. The date of the last search was 14 February 2017. There were no language restrictions.
Selection criteria: Randomized controlled trials of at least one year of MDI versus control for prevention of recurrent idiopathic (non-syndromic) nephrolithiasis in children.
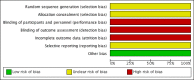
Data collection and analysis: We used standard methodologic procedures expected by Cochrane. Titles and abstracts were identified by search criteria and then screened for relevance, and then data extraction and risk of bias assessment were carried out. We assessed the quality of evidence using GRADE.
Main results: The search identified one study of 125 children (72 boys and 53 girls) with calcium-containing idiopathic nephrolithiasis and normal renal morphology following initial treatment with shockwave lithotripsy (SWL). Patients were randomized to oral potassium citrate 1 mEq/kg per day for 12 months versus no specific medication or preventive measure with results reported for a total of 96 patients (48 per group). This included children who were stone-free (n = 52) or had residual stone fragments (n = 44) following SWL. Primary outcomes:Medical therapy may lower rates of stone recurrence with a risk ratio (RR) of 0.19 (95% confidence interval (CI) 0.06 to 0.60; low quality evidence). This corresponds to 270 fewer stone recurrences per 1000 (133 fewer to 313 fewer) children. We downgraded the quality of evidence by two levels for very serious study limitations related to unclear allocation concealment (selection bias) and a high risk of performance, detection and attrition bias. While the data for adverse events were incomplete, they reported that six of 48 (12.5%) children receiving potassium citrate left the trial because of adverse effects. This corresponds to a RR of 13.0 (95% CI 0.75 to 224.53; very low quality evidence); an absolute effect size estimate could not be generated. We downgraded the quality of evidence for study limitations and imprecision.We found no information on retreatment rates.
Secondary outcomes: We found no evidence on serum electrolytes, 24-hour urine collection parameters or time to new stone formation.We were unable to perform any preplanned secondary analyses.
Authors' conclusions: Oral potassium citrate supplementation may reduce recurrent calcium urinary stone formation in children following SWL; however, our confidence in this finding is limited. A substantial number of children stopped the medication due to adverse events. There is no trial evidence on retreatment rates. There is a critical need for additional well-designed trials in children with nephrolithiasis.
Conflict of interest statement
AK: none known.
GG: none known.
HM: none known.
LB none known.
Figures



Update of
- doi: 10.1002/14651858.CD011252
Similar articles
-
Medical and surgical interventions for the treatment of urinary stones in children.Cochrane Database Syst Rev. 2018 Jun 2;6(6):CD010784. doi: 10.1002/14651858.CD010784.pub2. Cochrane Database Syst Rev. 2018. PMID: 29859007 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Non-pharmacological interventions for preventing delirium in hospitalised non-ICU patients.Cochrane Database Syst Rev. 2021 Jul 19;7(7):CD013307. doi: 10.1002/14651858.CD013307.pub2. Cochrane Database Syst Rev. 2021. PMID: 34280303 Free PMC article.
-
Interventions for infantile haemangiomas of the skin.Cochrane Database Syst Rev. 2018 Apr 18;4(4):CD006545. doi: 10.1002/14651858.CD006545.pub3. Cochrane Database Syst Rev. 2018. PMID: 29667726 Free PMC article.
-
Dietary interventions for recurrent abdominal pain in childhood.Cochrane Database Syst Rev. 2017 Mar 23;3(3):CD010972. doi: 10.1002/14651858.CD010972.pub2. Cochrane Database Syst Rev. 2017. PMID: 28334433 Free PMC article.
Cited by
-
Potassium Citrate is Better in Reducing Salt and Increasing Urine pH than Oral Intake of Lemonade: A Cross-Over Study.Med Sci Monit. 2018 Apr 1;24:1924-1929. doi: 10.12659/msm.909319. Med Sci Monit. 2018. PMID: 29605825 Free PMC article. Clinical Trial.
-
The current status of preventive measures for urinary calculi in children.Ther Adv Urol. 2021 Aug 14;13:17562872211039581. doi: 10.1177/17562872211039581. eCollection 2021 Jan-Dec. Ther Adv Urol. 2021. PMID: 34422114 Free PMC article. Review.
-
Role of Citrate in Pathophysiology and Medical Management of Bone Diseases.Nutrients. 2019 Oct 25;11(11):2576. doi: 10.3390/nu11112576. Nutrients. 2019. PMID: 31731473 Free PMC article. Review.
-
Scoping review of recent evidence on the management of pediatric urolithiasis: summary of meta-analyses, systematic reviews and relevant randomized controlled trials.Pediatr Surg Int. 2022 Oct;38(10):1349-1361. doi: 10.1007/s00383-022-05190-3. Epub 2022 Aug 8. Pediatr Surg Int. 2022. PMID: 35939126
-
Assessment of health equity consideration in Cochrane systematic reviews and primary studies on urolithiasis.Health Sci Rep. 2023 Feb 24;6(2):e1133. doi: 10.1002/hsr2.1133. eCollection 2023 Feb. Health Sci Rep. 2023. PMID: 36846534 Free PMC article.
References
References to studies included in this review
Sarica 2006 {published data only}
-
- Sarica K, Erturhan S, Yurtseven C, Yagci F. Effect of potassium citrate therapy on stone recurrence and regrowth after extracorporeal shockwave lithotripsy in children. Journal of Endourology / Endourological Society 2006;20(11):875‐9. - PubMed
References to studies excluded from this review
Choi 2011 {published data only}
-
- Choi JN, Lee JS, Shin JI. Low‐dose thiazide diuretics in children with idiopathic renal hypercalciuria. Acta Paediatrica 2011;100(8):71‐4. - PubMed
Gheissari 2012 {published data only}
IRCT2014041217234N1 {unpublished data only}
-
- IRCT2014041217234N1. Comparing effectiveness of combination of magnesium oxide and potassium citrate with potassium citrate in children with urolithiasis. www.irct.ir/searchresult.php?keyword=&id=17234&number=1&prt=... Date first received: 22 December 2014.
Naseri 2011 {published data only}
-
- Naseri M, Sadeghi R. Role of high‐dose hydrochlorothiazide in idiopathic hypercalciuric urolithiasis of childhood. Iranian Journal of Kidney Diseases 2011;5(3):162‐8. - PubMed
NCT00120731 {unpublished data only}
-
- NCT00120731. Effects of potassium citrate in urine of children with elevated calcium in urine and kidney stones. clinicaltrials.gov/ct2/show/study/NCT00120731 Date first received: 19 July 2005.
NCT02289755 {unpublished data only}
-
- NCT02289755. Evaluating ALLN‐177 for reducing urinary oxalate excretion in calcium oxalate kidney stone formers with hyperoxaluria. clinicaltrials.gov/ct2/show/study/NCT02289755 Date first received: 13 November 2014.
Ogûz 2013 {published data only}
-
- Ogûz U, Unsal A. The efficacy of medical prophylaxis in children with calcium oxalate urolithiasis after percutaneous nephrolithotomy. Journal of Endourology / Endourological Society 2013;27(1):92‐5. - PubMed
Tekin 2002 {published data only}
-
- Tekin A, Tekgul S, Atsu N, Bakkaloglu M, Kendi S. Oral potassium citrate treatment for idiopathic hypocitruria in children with calcium urolithiasis. Journal of Urology 2002;168(6):2572‐4. - PubMed
Yousefichaijan 2015 {published data only}
Additional references
Arrabal‐Polo 2013
-
- Arrabal‐Polo MA, Arrabal‐Martin M, Arias‐Santiago S, Garrido‐Gomez J, Poyatos‐Andujar A, Zuluaga‐Gomez A. Importance of citrate and the calcium: citrate ratio in patients with calcium renal lithiasis and severe lithogenesis. BJU International 2013;111(4):622‐7. [MEDLINE: ] - PubMed
Borghi 1996
-
- Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5‐year randomized prospective study. Journal of Urology 1996;155(3):839‐43. [MEDLINE: ] - PubMed
Bush 2010
-
- Bush NC, Xu L, Brown BJ, Holzer MS, Gingrich B, Schuler B, et al. Hospitalizations for pediatric stone disease in United States, 2002‐2007. Journal of Urology 2010;183(3):1151‐6. - PubMed
Dussol 2008
-
- Dussol B, Iovanna C, Rotily M, Morange S, Leonetti F, Dupuy P, et al. A randomized trial of low‐animal‐protein or high‐fiber diets for secondary prevention of calcium nephrolithiasis. Nephron Clinical Practice 2008;110(3):c185‐94. [PUBMED: 18957869] - PubMed
Dwyer 2012
EndNote [Computer program]
-
- Clarivate Analytics. EndNote. Version 7.6. Clarivate Analytics, 2016.
Escribano 2009
Escribano 2014
Fink 2013
-
- Fink HA, Wilt TJ, Eidman KE, Garimella PS, MacDonald R, Rutks IR, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Annals of Internal Medicine 2013;158(7):535‐43. [MEDLINE: ] - PubMed
Golder 2011
GRADEpro GDT [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 17 February 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Grimsby 2014
Guyatt 2008
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Schünemann HJ, et al. GRADE: what is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical Research Ed.) 2008;336(7651):995‐8. [DOI: 10.1136/bmj.39490.551019.BE] - DOI - PMC - PubMed
Guyatt 2011
Heilberg 2013
-
- Heilberg IP, Goldfarb DS. Optimum nutrition for kidney stone disease. Advances in Chronic Kidney Disease 2013;20(2):165‐74. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
-
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kairam 2013
-
- Kairam N, Allegra JR, Eskin B. Rise in emergency department visits of pediatric patients for renal colic from 1999 to 2008. Pediatric Emergency Care 2013;29(4):462‐4. [MEDLINE: ] - PubMed
Kocvara 1999
-
- Kocvara R, Plasgura P, Petrik A, Louzensky G, Bartonickova K, Dvoracek J. A prospective study of nonmedical prophylaxis after a first kidney stone. BJU International 1999;84(4):393‐8. [MEDLINE: ] - PubMed
Liberati 2009
Malek 1975
-
- Malek RS, Kelalis PP. Pediatric nephrolithiasis. Journal of Urology 1975;113(4):545‐51. [MEDLINE: ] - PubMed
Milliner 1993
-
- Milliner DS, Murphy ME. Urolithiasis in pediatric patients. Mayo Clinic Proceedings 1993;68(3):241‐8. [MEDLINE: ] - PubMed
Nimkin 1992
-
- Nimkin K, Lebowitz RL, Share JC, Teele RL. Urolithiasis in a children's hospital: 1985‐1990. Urologic Radiology 1992;14(3):139‐43. [MEDLINE: ] - PubMed
Pearle 2005
-
- Pearle MS, Calhoun EA, Curhan GC, Urologic Diseases of America Project. Urologic diseases in America project: urolithiasis. Journal of Urology 2005;173(3):848‐57. [MEDLINE: ] - PubMed
Pietrow 2002
-
- Pietrow PK, Pope JC 4th, Adams MC, Shyr Y, Brock JW 3rd. Clinical outcome of pediatric stone disease. Journal of Urology 2002;167(2 Pt 1):670‐3. [MEDLINE: ] - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rule 2014
Saxena 2010
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Seltzer 1996
-
- Seltzer MA, Low RK, McDonald M, Shami GS, Stoller ML. Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. Journal of Urology 1996;156(3):907‐9. [MEDLINE: ] - PubMed
Tasian 2017
Trinchieri 1996
-
- Trinchieri A. Epidemiology of urolithiasis. Archivio Italiano di Urologia, Andrologia 1996;68(4):203‐49. [MEDLINE: ] - PubMed
Troup 1972
-
- Troup CW, Lawnicki CC, Bourne RB, Hodgson NB. Renal calculus in children. Journal of Urology 1972;107(2):306‐7. [MEDLINE: ] - PubMed
VanDervoort 2007
-
- VanDervoort K, Wiesen J, Frank R, Vento S, Crosby V, Chandra M, et al. Urolithiasis in pediatric patients: a single center study of incidence, clinical presentation and outcome. Journal of Urology 2007;177(6):2300‐5. [MEDLINE: ] - PubMed
Walther 1980
-
- Walther PC, Lamm D, Kaplan GW. Pediatric urolithiases: a ten‐year review. Pediatrics 1980;65(6):1068‐72. [MEDLINE: ] - PubMed
Wells 2012
-
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 20 May 2014).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

