Biophysical properties of microvascular endothelium: Requirements for initiating and conducting electrical signals
- PMID: 29117630
- PMCID: PMC5809195
- DOI: 10.1111/micc.12429
Biophysical properties of microvascular endothelium: Requirements for initiating and conducting electrical signals
Abstract
Objective: Electrical signaling along the endothelium underlies spreading vasodilation and blood flow control. We use mathematical modeling to determine the electrical properties of the endothelium and gain insight into the biophysical determinants of electrical conduction.
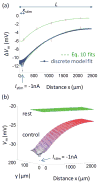
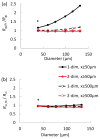
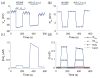
Methods: Electrical conduction data along endothelial tubes (40 μm wide, 2.5 mm long) isolated from mouse skeletal muscle resistance arteries were analyzed using cable equations and a multicellular computational model.
Results: Responses to intracellular current injection attenuate with an axial length constant (λ) of 1.2-1.4 mm. Data were fitted to estimate the axial (ra ; 10.7 MΩ/mm) and membrane (rm ; 14.5 MΩ∙mm) resistivities, EC membrane resistance (Rm ; 12 GΩ), and EC-EC coupling resistance (Rgj ; 4.5 MΩ) and predict that stimulation of ≥30 neighboring ECs is required to elicit 1 mV of hyperpolarization at distance = 2.5 mm. Opening Ca2+ -activated K+ channels (KCa ) along the endothelium reduced λ by up to 55%.
Conclusions: High Rm makes the endothelium sensitive to electrical stimuli and able to conduct these signals effectively. Whereas the activation of a group of ECs is required to initiate physiologically relevant hyperpolarization, this requirement is increased by myoendothelial coupling and KCa activation along the endothelium inhibits conduction by dissipating electrical signals.
Keywords: endothelium; membrane potential; membrane resistance; modeling; spreading vasodilation.
© 2017 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



References
-
- Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. - PubMed
-
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. - PubMed
-
- Segal SS, Duling BR. Communication between feed arteries and microvessels in hamster striated muscle: segmental vascular responses are functionally coordinated. Circ Res. 1986;59:283–290. - PubMed
-
- Cohen KD, Berg BR, Sarelius IH. Remote arteriolar dilations in response to muscle contraction under capillaries. Am J Physiol Heart Circ Physiol. 2000;278:H1916–1923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

