Redirecting T cells to hematological malignancies with bispecific antibodies
- PMID: 29118005
- PMCID: PMC5755042
- DOI: 10.1182/blood-2017-06-741058
Redirecting T cells to hematological malignancies with bispecific antibodies
Abstract
There is a need to improve outcomes for patients with recurrent and/or refractory hematological malignancies. Immunotherapy holds the promise to meet this need, because it does not rely on the cytotoxic mechanism of conventional therapies. Among different forms of immunotherapy, redirecting T cells to hematological malignancies with bispecific antibodies (BsAbs) is an attractive strategy. BsAbs are an "off-the-shelf" product that is easily scalable in contrast to adoptive T-cell therapies. Among these, the bispecific T-cell engager blinatumomab has emerged as the most successful BsAb to date. It consists of 2 single-chain variable fragments specific for CD19 present on B-cell malignancies and CD3 expressed on almost all T cells. Blinatumomab has shown potent antitumor activity as a single agent, particularly for acute lymphoblastic leukemia, resulting in its US Food and Drug Administration approval. However, although successful in inducing remissions, these are normally short-lived, with median response durations of <1 year. Nevertheless, the success of blinatumomab has reinvigorated the BsAb field, which is bustling with preclinical and clinical studies for not only B-cell-derived lymphoblastic leukemia and lymphoma but also acute myeloid leukemia and multiple myeloma. Here, we will review the successes and challenges of T-cell-targeted BsAbs for the immunotherapy of hematological malignancies with special focus on conducted clinical studies and strategies to improve their efficacy.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.P.V., C.L.B., and S.G. have patents and/or patent applications in the field of cancer immunotherapy. Pertinent to this review, M.P.V. and S.G. have one patent application entitled “Engager cells for Immunotherapy,” and C.L.B. and S.G. have one patent application entitled “Combination CD123 and C-type Lectin Molecule 1 Targeted T cells for Acute Myeloid Leukemia.” M.P.V. is a consultant/advisory board member for the Rally! Foundation. S.G. is a consultant/advisory board member for Merrimack.
Figures
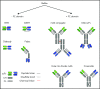
References
-
- Bargou R, Leo E, Zugmaier G, et al. . Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974-977. - PubMed
-
- Topp MS, Gökbuget N, Stein AS, et al. . Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57-66. - PubMed
-
- Turtle CJ, Riddell SR, Maloney DG. CD19-Targeted chimeric antigen receptor-modified T-cell immunotherapy for B-cell malignancies. Clin Pharmacol Ther. 2016;100(3):252-258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

