CTLA-4: a moving target in immunotherapy
- PMID: 29118008
- PMCID: PMC6317697
- DOI: 10.1182/blood-2017-06-741033
CTLA-4: a moving target in immunotherapy
Abstract
CD28 and CTLA-4 are members of a family of immunoglobulin-related receptors that are responsible for various aspects of T-cell immune regulation. The family includes CD28, CTLA-4, and ICOS as well as other proteins, including PD-1, BTLA, and TIGIT. These receptors have both stimulatory (CD28, ICOS) and inhibitory roles (CTLA-4, PD-1, BTLA, and TIGIT) in T-cell function. Increasingly, these pathways are targeted as part of immune modulatory strategies to treat cancers, referred to generically as immune checkpoint blockade, and conversely to treat autoimmunity and CTLA-4 deficiency. Here, we focus on the biology of the CD28/CTLA-4 pathway as a framework for understanding the impacts of therapeutic manipulation of this pathway.
© 2018 by The American Society of Hematology.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
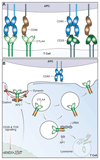
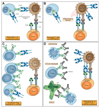
References
-
- Schwartz JC, Zhang X, Fedorov AA, Nathenson SG, Almo SC. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature. 2001;410(6828):604–608. - PubMed
-
- Stamper CC, Zhang Y, Tobin JF, et al. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410(6828):608–611. - PubMed
-
- Collins AV, Brodie DW, Gilbert RJC, et al. The Interaction Properties of Costimulatory Molecules Revisited. Immunity. 2002;17(2):201–210. - PubMed
-
- Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7(4):445–450. - PubMed
-
- Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11(12):852–863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

