Chronic inflammation is a feature of Achilles tendinopathy and rupture
- PMID: 29118051
- PMCID: PMC5867427
- DOI: 10.1136/bjsports-2017-098161
Chronic inflammation is a feature of Achilles tendinopathy and rupture
Abstract
Background: Recent investigation of human tissue and cells from positional tendons such as the rotator cuff has clarified the importance of inflammation in the development and progression of tendon disease. These mechanisms remain poorly understood in disease of energy-storing tendons such as the Achilles. Using tissue biopsies from patients, we investigated if inflammation is a feature of Achilles tendinopathy and rupture.
Methods: We studied Achilles tendon biopsies from symptomatic patients with either mid-portion tendinopathy or rupture for evidence of abnormal inflammatory signatures. Tendon-derived stromal cells from healthy hamstring and diseased Achilles were cultured to determine the effects of cytokine treatment on expression of inflammatory markers.
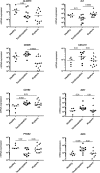
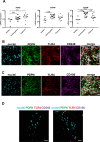
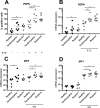
Results: Tendinopathic and ruptured Achilles highly expressed CD14+ and CD68+ cells and showed a complex inflammation signature, involving NF-κB, interferon and STAT-6 activation pathways. Interferon markers IRF1 and IRF5 were highly expressed in tendinopathic samples. Achilles ruptures showed increased PTGS2 and interleukin-8 expression. Tendinopathic and ruptured Achilles tissues expressed stromal fibroblast activation markers podoplanin and CD106. Tendon cells isolated from diseased Achilles showed increased expression of pro-inflammatory and stromal fibroblast activation markers after cytokine stimulation compared with healthy hamstring tendon cells.
Conclusions: Tissue and cells derived from tendinopathic and ruptured Achilles tendons show evidence of chronic (non-resolving) inflammation. The energy-storing Achilles shares common cellular and molecular inflammatory mechanisms with functionally distinct rotator cuff positional tendons. Differences seen in the profile of ruptured Achilles are likely to be attributable to a superimposed phase of acute inflammation and neo-vascularisation. Strategies that target chronic inflammation are of potential therapeutic benefit for patients with Achilles tendon disease.
Keywords: Achilles tendon; immunology; orthopaedics; tendinopathy; tendon.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures


Similar articles
-
The shift in macrophages polarisation after tendon injury: A systematic review.J Orthop Translat. 2019 Dec 24;21:24-34. doi: 10.1016/j.jot.2019.11.009. eCollection 2020 Mar. J Orthop Translat. 2019. PMID: 32071872 Free PMC article. Review.
-
Persistent stromal fibroblast activation is present in chronic tendinopathy.Arthritis Res Ther. 2017 Jan 25;19(1):16. doi: 10.1186/s13075-016-1218-4. Arthritis Res Ther. 2017. PMID: 28122639 Free PMC article.
-
Stereological quantification of immune-competent cells in baseline biopsy specimens from achilles tendons: results from patients with chronic tendinopathy followed for more than 4 years.Am J Sports Med. 2014 Oct;42(10):2435-45. doi: 10.1177/0363546514542329. Epub 2014 Jul 31. Am J Sports Med. 2014. PMID: 25081311
-
15-Epi-LXA4 and MaR1 counter inflammation in stromal cells from patients with Achilles tendinopathy and rupture.FASEB J. 2019 Jul;33(7):8043-8054. doi: 10.1096/fj.201900196R. Epub 2019 Mar 27. FASEB J. 2019. PMID: 30916999 Free PMC article.
-
Chronic Achilles Tendon Disorders: Tendinopathy and Chronic Rupture.Clin Sports Med. 2015 Oct;34(4):607-24. doi: 10.1016/j.csm.2015.06.010. Epub 2015 Jul 31. Clin Sports Med. 2015. PMID: 26409586 Review.
Cited by
-
Association of TNF-α -308G > A polymorphism with susceptibility to tendinopathy in athletes: a case-control study.BMC Sports Sci Med Rehabil. 2021 May 13;13(1):51. doi: 10.1186/s13102-021-00276-2. BMC Sports Sci Med Rehabil. 2021. PMID: 33985554 Free PMC article.
-
Effect of Acellular Amnion With Increased TGF-β and bFGF Levels on the Biological Behavior of Tenocytes.Front Bioeng Biotechnol. 2020 May 14;8:446. doi: 10.3389/fbioe.2020.00446. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32478059 Free PMC article.
-
The shift in macrophages polarisation after tendon injury: A systematic review.J Orthop Translat. 2019 Dec 24;21:24-34. doi: 10.1016/j.jot.2019.11.009. eCollection 2020 Mar. J Orthop Translat. 2019. PMID: 32071872 Free PMC article. Review.
-
Adenylate cyclase activates the cAMP signalling pathway to enhance platelet-rich plasma-treated Achilles tendon disease, a theoretical bioinformatics-based study.World J Orthop. 2024 Feb 18;15(2):192-200. doi: 10.5312/wjo.v15.i2.192. eCollection 2024 Feb 18. World J Orthop. 2024. PMID: 38464349 Free PMC article.
-
Network pharmacology integrated with experimental validation revealed the anti-inflammatory effects of Andrographis paniculata.Sci Rep. 2021 May 7;11(1):9752. doi: 10.1038/s41598-021-89257-6. Sci Rep. 2021. PMID: 33963245 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
