Valproic Acid Sensitizes Hepatocellular Carcinoma Cells to Proton Therapy by Suppressing NRF2 Activation
- PMID: 29118323
- PMCID: PMC5678087
- DOI: 10.1038/s41598-017-15165-3
Valproic Acid Sensitizes Hepatocellular Carcinoma Cells to Proton Therapy by Suppressing NRF2 Activation
Erratum in
-
Publisher Correction: Valproic Acid Sensitizes Hepatocellular Carcinoma Cells to Proton Therapy by Suppressing NRF2 Activation.Sci Rep. 2018 May 10;8(1):7597. doi: 10.1038/s41598-018-25326-7. Sci Rep. 2018. PMID: 29748578 Free PMC article.
Abstract
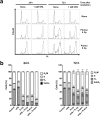
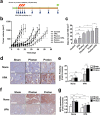
Although efficacy of combined histone deacetylase (HDAC) inhibitors and conventional photon radiotherapy is being tested in clinical trials, their combined effect with proton beam radiotherapy has yet to be determined. Here, we compared combined effect of valproic acid (VPA), a class I and II HDAC inhibitor and antiepileptic drug with proton and photon irradiation in hepatocellular carcinoma (HCC) cells in vitro and in vivo. We found that VPA sensitized more Hep3B cells to proton than to photon irradiation. VPA prolonged proton-induced DNA damage and augmented proton-induced apoptosis. In addition, VPA further increased proton-induced production of intracellular reactive oxygen species and suppressed expression of nuclear factor erythroid-2-related factor 2 (NRF2), a key transcription factor regulating antioxidant response. Downregulation of NRF2 by siRNA transfection increased proton-induced apoptotic cell death, supporting NRF2 as a target of VPA in radiosensitization. In Hep3B tumor xenograft models, VPA significantly enhanced proton-induced tumor growth delay with increased apoptosis and decreased NRF2 expression in vivo. Collectively, our study highlights a proton radiosensitizing effect of VPA in HCC cells. As NRF2 is an emerging prognostic marker contributing to radioresistance in HCC, targeting NRF2 pathway may impact clinical outcome of proton beam radiotherapy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

