Impaired development of the cerebral cortex in infants with congenital heart disease is correlated to reduced cerebral oxygen delivery
- PMID: 29118365
- PMCID: PMC5678433
- DOI: 10.1038/s41598-017-14939-z
Impaired development of the cerebral cortex in infants with congenital heart disease is correlated to reduced cerebral oxygen delivery
Abstract
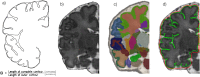
Neurodevelopmental impairment is the most common comorbidity associated with complex congenital heart disease (CHD), while the underlying biological mechanism remains unclear. We hypothesised that impaired cerebral oxygen delivery in infants with CHD is a cause of impaired cortical development, and predicted that cardiac lesions most associated with reduced cerebral oxygen delivery would demonstrate the greatest impairment of cortical development. We compared 30 newborns with complex CHD prior to surgery and 30 age-matched healthy controls using brain MRI. The cortex was assessed using high resolution, motion-corrected T2-weighted images in natural sleep, analysed using an automated pipeline. Cerebral oxygen delivery was calculated using phase contrast angiography and pre-ductal pulse oximetry, while regional cerebral oxygen saturation was estimated using near-infrared spectroscopy. We found that impaired cortical grey matter volume and gyrification index in newborns with complex CHD was linearly related to reduced cerebral oxygen delivery, and that cardiac lesions associated with the lowest cerebral oxygen delivery were associated with the greatest impairment of cortical development. These findings suggest that strategies to improve cerebral oxygen delivery may help reduce brain dysmaturation in newborns with CHD, and may be most relevant for children with CHD whose cardiac defects remain unrepaired for prolonged periods after birth.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

