The Origins and Functions of Tissue-Resident Macrophages in Kidney Development
- PMID: 29118719
- PMCID: PMC5660965
- DOI: 10.3389/fphys.2017.00837
The Origins and Functions of Tissue-Resident Macrophages in Kidney Development
Abstract
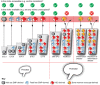
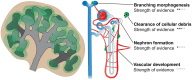
The adult kidney hosts tissue-resident macrophages that can cause, prevent, and/or repair renal damage. Most of these macrophages derive from embryonic progenitors that colonize the kidney during its development and proliferate in situ throughout adulthood. Although the precise origins of kidney macrophages remain controversial, recent studies have revealed that embryonic macrophage progenitors initially migrate from the yolk sac, and later from the fetal liver, into the developing kidney. Once in the kidney, tissue-specific transcriptional regulators specify macrophage progenitors into dedicated kidney macrophages. Studies suggest that kidney macrophages facilitate many processes during renal organogenesis, such as branching morphogenesis and the clearance of cellular debris; however, little is known about how the origins and specification of kidney macrophages dictate their function. Here, we review significant new findings about the origins, specification, and developmental functions of kidney macrophages.
Keywords: angiogenesis; branching morphogenesis; metanephros; monocyte; nephron; ontogeny; phagocyte; renal.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

