Redox Regulation of Inflammatory Processes Is Enzymatically Controlled
- PMID: 29118897
- PMCID: PMC5651112
- DOI: 10.1155/2017/8459402
Redox Regulation of Inflammatory Processes Is Enzymatically Controlled
Abstract
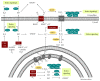
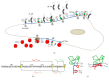
Redox regulation depends on the enzymatically controlled production and decay of redox active molecules. NADPH oxidases, superoxide dismutases, nitric oxide synthases, and others produce the redox active molecules superoxide, hydrogen peroxide, nitric oxide, and hydrogen sulfide. These react with target proteins inducing spatiotemporal modifications of cysteine residues within different signaling cascades. Thioredoxin family proteins are key regulators of the redox state of proteins. They regulate the formation and removal of oxidative modifications by specific thiol reduction and oxidation. All of these redox enzymes affect inflammatory processes and the innate and adaptive immune response. Interestingly, this regulation involves different mechanisms in different biological compartments and specialized cell types. The localization and activity of distinct proteins including, for instance, the transcription factor NFκB and the immune mediator HMGB1 are redox-regulated. The transmembrane protein ADAM17 releases proinflammatory mediators, such as TNFα, and is itself regulated by a thiol switch. Moreover, extracellular redox enzymes were shown to modulate the activity and migration behavior of various types of immune cells by acting as cytokines and/or chemokines. Within this review article, we will address the concept of redox signaling and the functions of both redox enzymes and redox active molecules in innate and adaptive immune responses.
Figures



References
-
- Hanschmann E.-M., Godoy J. R., Berndt C., Hudemann C., Lillig C. H. Thioredoxins, glutaredoxins, and peroxiredoxins—molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxidants & Redox Signaling. 2013;19(13):1539–1605. doi: 10.1089/ars.2012.4599. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

