Comparing the physiochemical parameters of three celluloses reveals new insights into substrate suitability for fungal enzyme production
- PMID: 29119000
- PMCID: PMC5669031
- DOI: 10.1186/s40694-017-0039-9
Comparing the physiochemical parameters of three celluloses reveals new insights into substrate suitability for fungal enzyme production
Abstract
Background: The industrial applications of cellulases are mostly limited by the costs associated with their production. Optimized production pathways are therefore desirable. Based on their enzyme inducing capacity, celluloses are commonly used in fermentation media. However, the influence of their physiochemical characteristics on the production process is not well understood. In this study, we examined how physical, structural and chemical properties of celluloses influence cellulase and hemicellulase production in an industrially-optimized and a non-engineered filamentous fungus: Trichoderma reesei RUT-C30 and Neurospora crassa. The performance was evaluated by quantifying gene induction, protein secretion and enzymatic activities.
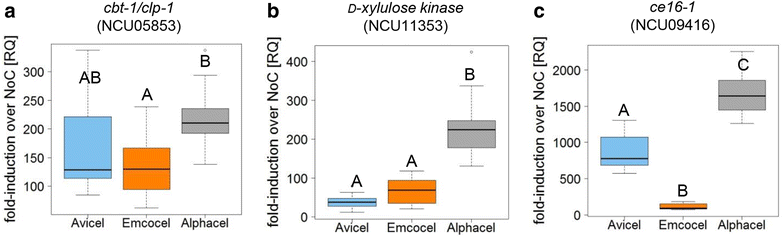
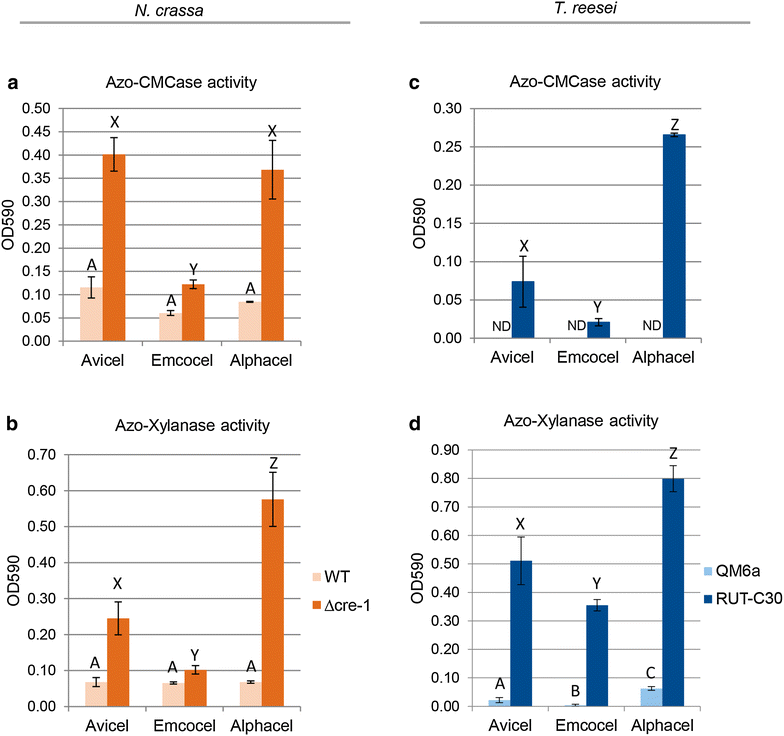
Results: Among the three investigated substrates, the powdered cellulose was found to be the most impure, and the residual hemicellulosic content was efficiently perceived by the fungi. It was furthermore found to be the least crystalline substrate and consequently was the most readily digested cellulose in vitro. In vivo however, only RUT-C30 was able to take full advantage of these factors. When comparing carbon catabolite repressed and de-repressed strains of T. reesei and N. crassa, we found that cre1/cre-1 is at least partially responsible for this observation, but that the different wiring of the molecular signaling networks is also relevant.
Conclusions: Our findings indicate that crystallinity and hemicellulose content are major determinants of performance. Moreover, the genetic background between WT and modified strains greatly affects the ability to utilize the cellulosic substrate. By highlighting key factors to consider when choosing the optimal cellulosic product for enzyme production, this study has relevance for the optimization of a critical step in the biotechnological (hemi-) cellulase production process.
Keywords: Cellulase production; Cellulose crystallinity; Microcrystalline cellulose; Neurospora crassa; Powdered cellulose; RUT-C30; Trichoderma reesei.
Figures





References
-
- Report. Technical enzymes market by type (cellulases, amylases, proteases, lipases, other enzymes), application (bioethanol, paper and pulp, textile and leather, starch processing, other applications), and by region—global forecasts to 2021. Research and markets. 2016.
LinkOut - more resources
Full Text Sources
Other Literature Sources

