Re-evaluation of protein kinase CK2 pleiotropy: new insights provided by a phosphoproteomics analysis of CK2 knockout cells
- PMID: 29119230
- PMCID: PMC11105740
- DOI: 10.1007/s00018-017-2705-8
Re-evaluation of protein kinase CK2 pleiotropy: new insights provided by a phosphoproteomics analysis of CK2 knockout cells
Abstract
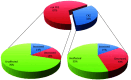
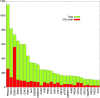
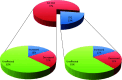
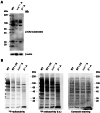
CK2 denotes a ubiquitous and pleiotropic protein kinase whose holoenzyme is composed of two catalytic (α and/or α') and two regulatory β subunits. The CK2 consensus sequence, S/T-x-x-D/E/pS/pT is present in numerous phosphosites, but it is not clear how many of these are really generated by CK2. To gain information about this issue, advantage has been taken of C2C12 cells entirely deprived of both CK2 catalytic subunits by the CRISPR/Cas9 methodology. A comparative SILAC phosphoproteomics analysis reveals that, although about 30% of the quantified phosphosites do conform to the CK2 consensus, only one-third of these are substantially reduced in the CK2α/α'(-/-) cells, consistent with their generation by CK2. A parallel study with C2C12 cells deprived of the regulatory β subunit discloses a role of this subunit in determining CK2 targeting. We also find that phosphosites notoriously generated by CK2 are not fully abrogated in CK2α/α'(-/-) cells, while some phosphosites unrelated to CK2 are significantly altered. Collectively taken our data allow to conclude that the phosphoproteome generated by CK2 is not as ample and rigidly pre-determined as it was believed before. They also show that the lack of CK2 promotes phosphoproteomics perturbations attributable to kinases other than CK2.
Keywords: Cell signalling; Kinase inhibitors; Protein phosphorylation; Quantitative proteomics; Sequence logo.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

