Endoplasmic Reticulum Stress and Allergic Diseases
- PMID: 29119328
- PMCID: PMC5683051
- DOI: 10.1007/s11882-017-0751-9
Endoplasmic Reticulum Stress and Allergic Diseases
Abstract
Purpose of review: In this review, we will integrate recent knowledge on endoplasmic reticulum (ER) stress and allergy, thereby highlighting the therapeutic potential of ER stress in the context of precision medicine for allergic diseases.
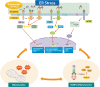
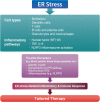
Recent findings: Emerging evidence suggests that allergic diseases are very heterogeneous having numerous endotypes. This leads to the new era of modern medicine, which assumes that a particular endotype-driven therapy, called precision medicine, would be more efficacious in a specific group of patients rather than in all patients. Currently, a dichotomy involving type 2/non-type 2 immune response underlies most of the studies on inflammatory and immunologic mechanisms of allergic disorders. Whereas there are several approved or investigational endotype-driven therapeutic agents targeting type 2 immune responses, investigation of mechanisms and endotype-driven interventions regarding non-type 2 immune response lags far behind. Considering that non-type 2 immune response may represent a significant proportion of allergic disease, particularly corticosteroid-resistant severe disease, defining a novel concept of endotype-driven approach may be essential. Recently, stress responses originate from the endoplasmic reticulum (ER) and the associated inflammatory molecular platform has been suggested as a crucial player of immune and inflammatory responses. This implies that ER stress-related pathways may represent a new endotype-driven therapeutic strategy in the treatment of allergic diseases.
Keywords: Allergic diseases; Biomarker; ER stress; Endoplasmic reticulum stress; Inflammation; Precision medicine.
Conflict of interest statement
Conflict of Interest
Dr. Lee reports grants from The Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea, grants from The Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, Information and Communication Technology and Future Planning, grants from Biomedical Research Institute, Chonbuk National University Hospital, during the conduct of the study. The other authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Figures
References
-
- Muraro A, Lemanske RF, Jr, Hellings PW, Akdis CA, Bieber T, Casale TB, et al. Precision medicine in patients with allergic diseases: airway diseases and atopic dermatitis-PRACTALL document of the European academy of allergy and clinical immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2016;137(5):1347–1358. doi: 10.1016/j.jaci.2016.03.010. - DOI - PubMed
-
- Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. 10.1067/mcp.2001.113989. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

