Rotavirus infection
- PMID: 29119972
- PMCID: PMC5858916
- DOI: 10.1038/nrdp.2017.83
Rotavirus infection
Abstract
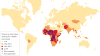
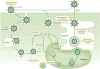
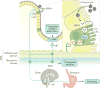
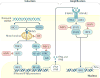
Rotavirus infections are a leading cause of severe, dehydrating gastroenteritis in children <5 years of age. Despite the global introduction of vaccinations for rotavirus over a decade ago, rotavirus infections still result in >200,000 deaths annually, mostly in low-income countries. Rotavirus primarily infects enterocytes and induces diarrhoea through the destruction of absorptive enterocytes (leading to malabsorption), intestinal secretion stimulated by rotavirus non-structural protein 4 and activation of the enteric nervous system. In addition, rotavirus infections can lead to antigenaemia (which is associated with more severe manifestations of acute gastroenteritis) and viraemia, and rotavirus can replicate in systemic sites, although this is limited. Reinfections with rotavirus are common throughout life, although the disease severity is reduced with repeat infections. The immune correlates of protection against rotavirus reinfection and recovery from infection are poorly understood, although rotavirus-specific immunoglobulin A has a role in both aspects. The management of rotavirus infection focuses on the prevention and treatment of dehydration, although the use of antiviral and anti-emetic drugs can be indicated in some cases.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Bishop RF, et al. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973;2:1281–1283. This study shows visualization of rotavirus as the causative agent of viral gastroenteritis and the associated histopathology. - PubMed
-
- Flewett TH, et al. Letter: Virus particles in gastroenteritis. Lancet. 1973;2:1497. - PubMed
-
- Estes MK, Greenberg HB. In: Field’s Virology. Knipe DM, Howley PM, editors. Lippincott: Williams & Wilkins; 2013. pp. 1347–1401.
-
- Matthijnssens J, et al. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch Virol. 2012;157:1177–1182. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

