Tumor conditions induce bone marrow expansion of granulocytic, but not monocytic, immunosuppressive leukocytes with increased CXCR2 expression in mice
- PMID: 29120053
- PMCID: PMC5877118
- DOI: 10.1002/eji.201746976
Tumor conditions induce bone marrow expansion of granulocytic, but not monocytic, immunosuppressive leukocytes with increased CXCR2 expression in mice
Abstract
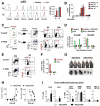
Myeloid-derived suppressor cells (MDSCs) promote tumor growth through, in part, inhibiting T-cell immunity. However, mechanisms underlying MDSC expansion and guidance of MDSCs toward the tumor microenvironment remain unclear. Employing Percoll density gradients, we separate bone marrow (BM) leukocytes from tumor-bearing mice into four density-increasing bands with myeloid leukocytes enriched in bands III and IV. Band III comprises monocytes and low-density granulocytes, both confirmed to be M-MDSCs and G-MDSCs, respectively, by displaying potent inhibition of T-cell proliferation. However, monocytes act as M-MDSCs not only under tumor conditions but also the healthy condition. In contrast, band IV contains non-inhibitory, mature granulocytes. Only band III G-MDSCs display significant expansion in mice bearing B16 melanoma, Lewis lung carcinoma, or MC38 colon carcinoma. The expanded G-MDSCs also show increased CXCR2 expression, which guides egress out of BM, and produce arginase-1 and ROS upon encountering antigen-activated T cells. Adoptive transfer assays demonstrate that both G-MDSCs and mature granulocytes infiltrate tumors, but only the former displays sustention and accumulation. Intratumoral administrations of granulocytes further demonstrate that G-MDSCs promote tumor growth, whereas mature granulocytes exert minimal effects, or execute powerful anti-tumor effects providing the presence of PMN activation mechanisms in the tumor microenvironment.
Keywords: Bone marrow PMN; Ly6C; Melanoma; Myeloid-derived suppressor cells; Myelopoiesis.
© 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Figures



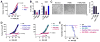
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

