The liquid structure of elastin
- PMID: 29120326
- PMCID: PMC5703643
- DOI: 10.7554/eLife.26526
The liquid structure of elastin
Abstract
The protein elastin imparts extensibility, elastic recoil, and resilience to tissues including arterial walls, skin, lung alveoli, and the uterus. Elastin and elastin-like peptides are hydrophobic, disordered, and undergo liquid-liquid phase separation upon self-assembly. Despite extensive study, the structure of elastin remains controversial. We use molecular dynamics simulations on a massive scale to elucidate the structural ensemble of aggregated elastin-like peptides. Consistent with the entropic nature of elastic recoil, the aggregated state is stabilized by the hydrophobic effect. However, self-assembly does not entail formation of a hydrophobic core. The polypeptide backbone forms transient, sparse hydrogen-bonded turns and remains significantly hydrated even as self-assembly triples the extent of non-polar side chain contacts. Individual chains in the assembly approach a maximally-disordered, melt-like state which may be called the liquid state of proteins. These findings resolve long-standing controversies regarding elastin structure and function and afford insight into the phase separation of disordered proteins.
Keywords: biophysics; intrinsically-disordered protein; molecular dynamics; none; phase separation; protein aggregation; self-assembled elastomer; structural biology.
Conflict of interest statement
No competing interests declared.
Figures

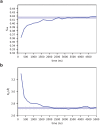
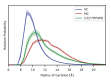
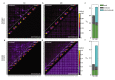
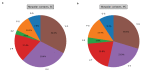









Similar articles
-
Role of Liquid-Liquid Phase Separation in Assembly of Elastin and Other Extracellular Matrix Proteins.J Mol Biol. 2018 Nov 2;430(23):4741-4753. doi: 10.1016/j.jmb.2018.06.010. Epub 2018 Jun 8. J Mol Biol. 2018. PMID: 29886015 Review.
-
Direct observation of structure and dynamics during phase separation of an elastomeric protein.Proc Natl Acad Sci U S A. 2017 May 30;114(22):E4408-E4415. doi: 10.1073/pnas.1701877114. Epub 2017 May 15. Proc Natl Acad Sci U S A. 2017. PMID: 28507126 Free PMC article.
-
Proline periodicity modulates the self-assembly properties of elastin-like polypeptides.J Biol Chem. 2010 Dec 17;285(51):39779-89. doi: 10.1074/jbc.M110.164467. Epub 2010 Oct 13. J Biol Chem. 2010. PMID: 20947499 Free PMC article.
-
Kinetics and morphology of self-assembly of an elastin-like polypeptide based on the alternating domain arrangement of human tropoelastin.Biochemistry. 2010 Jul 13;49(27):5726-33. doi: 10.1021/bi100468v. Biochemistry. 2010. PMID: 20527981
-
Self-assembling systems comprising intrinsically disordered protein polymers like elastin-like recombinamers.J Pept Sci. 2022 Jan;28(1):e3362. doi: 10.1002/psc.3362. Epub 2021 Sep 20. J Pept Sci. 2022. PMID: 34545666 Review.
Cited by
-
IM30 IDPs form a membrane-protective carpet upon super-complex disassembly.Commun Biol. 2020 Oct 21;3(1):595. doi: 10.1038/s42003-020-01314-4. Commun Biol. 2020. PMID: 33087858 Free PMC article.
-
Elastin is heterogeneously cross-linked.J Biol Chem. 2018 Sep 28;293(39):15107-15119. doi: 10.1074/jbc.RA118.004322. Epub 2018 Aug 14. J Biol Chem. 2018. PMID: 30108173 Free PMC article.
-
Choosing the right density for a concentrated protein system like gluten in a coarse-grained model.Eur Biophys J. 2023 Oct;52(6-7):583-591. doi: 10.1007/s00249-023-01667-8. Epub 2023 Jun 28. Eur Biophys J. 2023. PMID: 37378869 Free PMC article.
-
Seeing Keratinocyte Proteins through the Looking Glass of Intrinsic Disorder.Int J Mol Sci. 2021 Jul 24;22(15):7912. doi: 10.3390/ijms22157912. Int J Mol Sci. 2021. PMID: 34360678 Free PMC article. Review.
-
Engineering the Architecture of Elastin-Like Polypeptides: From Unimers to Hierarchical Self-Assembly.Adv Ther (Weinh). 2020 Mar;3(3):1900164. doi: 10.1002/adtp.201900164. Epub 2020 Feb 3. Adv Ther (Weinh). 2020. PMID: 34307837 Free PMC article.
References
-
- Andrady AL, Mark JE. Thermoelasticity of swollen elastin networks at constant composition. Biopolymers. 1980;19:849–855. doi: 10.1002/bip.1980.360190410. - DOI
-
- Bellingham CM, Woodhouse KA, Robson P, Rothstein SJ, Keeley FW. Self-aggregation characteristics of recombinantly expressed human elastin polypeptides. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 2001;1550:6–19. doi: 10.1016/S0167-4838(01)00262-X. - DOI - PubMed
-
- Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR. Molecular dynamics with coupling to an external bath. Journal of Chemical Physics. 1984;81:3684–3690. doi: 10.1063/1.448118. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

