Amorphous Solid Dispersion of Epigallocatechin Gallate for Enhanced Physical Stability and Controlled Release
- PMID: 29120370
- PMCID: PMC5748645
- DOI: 10.3390/ph10040088
Amorphous Solid Dispersion of Epigallocatechin Gallate for Enhanced Physical Stability and Controlled Release
Abstract
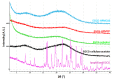
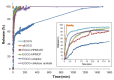
Epigallocatechin gallate (EGCG) has been recognized as the most prominent green tea extract due to its healthy influences. The high instability and low bioavailability, however, strongly limit its utilization in food and drug industries. This work, for the first time, develops amorphous solid dispersion of EGCG to enhance its bioavailability and physical stability. Four commonly used polymeric excipients are found to be compatible with EGCG in water-dioxane mixtures via a stepwise mixing method aided by vigorous mechanical interference. The dispersions are successfully generated by lyophilization. The physical stability of the dispersions is significantly improved compared to pure amorphous EGCG in stress condition (elevated temperature and relative humidity) and simulated gastrointestinal tract environment. From the drug release tests, one of the dispersions, EGCG-Soluplus® 50:50 (w/w) shows a dissolution profile that only 50% EGCG is released in the first 20 min, and the remains are slowly released in 24 h. This sustained release profile may open up new possibilities to increase EGCG bioavailability via extending its elimination time in plasma.
Keywords: amorphous solid dispersion; bioavailability; controlled release; epigallocatechin gallate; physical stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
Pharmacokinetic, toxicokinetic, and bioavailability studies of epigallocatechin-3-gallate loaded solid lipid nanoparticle in rat model.Drug Dev Ind Pharm. 2019 Sep;45(9):1506-1514. doi: 10.1080/03639045.2019.1634091. Epub 2019 Jul 2. Drug Dev Ind Pharm. 2019. PMID: 31215261
-
Bioavailability of Epigallocatechin Gallate Administered With Different Nutritional Strategies in Healthy Volunteers.Antioxidants (Basel). 2020 May 19;9(5):440. doi: 10.3390/antiox9050440. Antioxidants (Basel). 2020. PMID: 32438698 Free PMC article.
-
Stability of glycosylated complexes loaded with Epigallocatechin 3-gallate (EGCG).Food Chem. 2023 Jun 1;410:135364. doi: 10.1016/j.foodchem.2022.135364. Epub 2022 Dec 29. Food Chem. 2023. PMID: 36623458
-
Advanced Nanovehicles-Enabled Delivery Systems of Epigallocatechin Gallate for Cancer Therapy.Front Chem. 2020 Oct 23;8:573297. doi: 10.3389/fchem.2020.573297. eCollection 2020. Front Chem. 2020. PMID: 33195062 Free PMC article. Review.
-
Advances in the Antagonism of Epigallocatechin-3-gallate in the Treatment of Digestive Tract Tumors.Molecules. 2019 May 3;24(9):1726. doi: 10.3390/molecules24091726. Molecules. 2019. PMID: 31058847 Free PMC article. Review.
Cited by
-
Stability, Morphology, and Effects of In Vitro Digestion on the Antioxidant Properties of Polyphenol Inclusion Complexes with β-Cyclodextrin.Molecules. 2022 Jun 14;27(12):3808. doi: 10.3390/molecules27123808. Molecules. 2022. PMID: 35744933 Free PMC article.
-
The Changes in Antioxidant Activity of Selected Flavonoids and Caffeine Depending on the Dosage and Form of Thiamine.Molecules. 2021 Aug 3;26(15):4702. doi: 10.3390/molecules26154702. Molecules. 2021. PMID: 34361853 Free PMC article.
-
Proximate, Elemental, and Functional Properties of Novel Solid Dispersions of Moringa oleifera Leaf Powder.Molecules. 2022 Aug 3;27(15):4935. doi: 10.3390/molecules27154935. Molecules. 2022. PMID: 35956885 Free PMC article.
-
Whey Protein Sodium-Caseinate as a Deliverable Vector for EGCG: In Vitro Optimization of Its Bioaccessibility, Bioavailability, and Bioactivity Mode of Actions.Molecules. 2024 May 31;29(11):2588. doi: 10.3390/molecules29112588. Molecules. 2024. PMID: 38893466 Free PMC article.
-
Amorphous Pterostilbene Delivery Systems Preparation-Innovative Approach to Preparation Optimization.Pharmaceutics. 2023 Apr 13;15(4):1231. doi: 10.3390/pharmaceutics15041231. Pharmaceutics. 2023. PMID: 37111715 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

