Ultra-selective looming detection from radial motion opponency
- PMID: 29120418
- PMCID: PMC7457385
- DOI: 10.1038/nature24626
Ultra-selective looming detection from radial motion opponency
Abstract
Nervous systems combine lower-level sensory signals to detect higher-order stimulus features critical to survival, such as the visual looming motion created by an imminent collision or approaching predator. Looming-sensitive neurons have been identified in diverse animal species. Different large-scale visual features such as looming often share local cues, which means loom-detecting neurons face the challenge of rejecting confounding stimuli. Here we report the discovery of an ultra-selective looming detecting neuron, lobula plate/lobula columnar, type II (LPLC2) in Drosophila, and show how its selectivity is established by radial motion opponency. In the fly visual system, directionally selective small-field neurons called T4 and T5 form a spatial map in the lobula plate, where they each terminate in one of four retinotopic layers, such that each layer responds to motion in a different cardinal direction. Single-cell anatomical analysis reveals that each arm of the LPLC2 cross-shaped primary dendrites ramifies in one of these layers and extends along that layer's preferred motion direction. In vivo calcium imaging demonstrates that, as their shape predicts, individual LPLC2 neurons respond strongly to outward motion emanating from the centre of the neuron's receptive field. Each dendritic arm also receives local inhibitory inputs directionally selective for inward motion opposing the excitation. This radial motion opponency generates a balance of excitation and inhibition that makes LPLC2 non-responsive to related patterns of motion such as contraction, wide-field rotation or luminance change. As a population, LPLC2 neurons densely cover visual space and terminate onto the giant fibre descending neurons, which drive the jump muscle motor neuron to trigger an escape take off. Our findings provide a mechanistic description of the selective feature detection that flies use to discern and escape looming threats.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


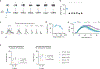





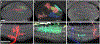


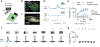

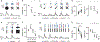

Comment in
-
Collision Avoidance: Broadening the Toolkit for Directionally Selective Motion Computations.Curr Biol. 2018 Feb 5;28(3):R124-R126. doi: 10.1016/j.cub.2017.12.010. Curr Biol. 2018. PMID: 29408261
References
-
- Lettvin JY, Maturana HR, McCulloch WS & Pitts WH What the frog’s eye tells the frog’s brain. Proceedings of the IRE 47, 1940–1951 (1959).
-
- Gibson JJ Visually controlled locomotion and visual orientation in animals. British journal of psychology 49, 182–194 (1958). - PubMed
-
- O’Shea M & Williams J The anatomy and output connection of a locust visual interneurone; the lobular giant movement detector (LGMD) neurone. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology 91, 257–266 (1974).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

