Discovery and Optimization of Potent, Cell-Active Pyrazole-Based Inhibitors of Lactate Dehydrogenase (LDH)
- PMID: 29120638
- PMCID: PMC5894102
- DOI: 10.1021/acs.jmedchem.7b00941
Discovery and Optimization of Potent, Cell-Active Pyrazole-Based Inhibitors of Lactate Dehydrogenase (LDH)
Abstract
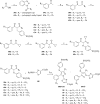
We report the discovery and medicinal chemistry optimization of a novel series of pyrazole-based inhibitors of human lactate dehydrogenase (LDH). Utilization of a quantitative high-throughput screening paradigm facilitated hit identification, while structure-based design and multiparameter optimization enabled the development of compounds with potent enzymatic and cell-based inhibition of LDH enzymatic activity. Lead compounds such as 63 exhibit low nM inhibition of both LDHA and LDHB, submicromolar inhibition of lactate production, and inhibition of glycolysis in MiaPaCa2 pancreatic cancer and A673 sarcoma cells. Moreover, robust target engagement of LDHA by lead compounds was demonstrated using the cellular thermal shift assay (CETSA), and drug-target residence time was determined via SPR. Analysis of these data suggests that drug-target residence time (off-rate) may be an important attribute to consider for obtaining potent cell-based inhibition of this cancer metabolism target.
Conflict of interest statement
The authors declare no competing financial interest
Figures
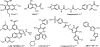
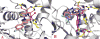

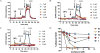

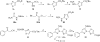


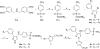



Similar articles
-
Pyrazole-Based Lactate Dehydrogenase Inhibitors with Optimized Cell Activity and Pharmacokinetic Properties.J Med Chem. 2020 Oct 8;63(19):10984-11011. doi: 10.1021/acs.jmedchem.0c00916. Epub 2020 Sep 27. J Med Chem. 2020. PMID: 32902275 Free PMC article.
-
Synthesis and biological characterization of an orally bioavailable lactate dehydrogenase-A inhibitor against pancreatic cancer.Eur J Med Chem. 2024 Sep 5;275:116598. doi: 10.1016/j.ejmech.2024.116598. Epub 2024 Jun 17. Eur J Med Chem. 2024. PMID: 38925013 Free PMC article.
-
Optimization of ether and aniline based inhibitors of lactate dehydrogenase.Bioorg Med Chem Lett. 2021 Jun 1;41:127974. doi: 10.1016/j.bmcl.2021.127974. Epub 2021 Mar 24. Bioorg Med Chem Lett. 2021. PMID: 33771585 Free PMC article.
-
Thiazole-containing compounds as therapeutic targets for cancer therapy.Eur J Med Chem. 2020 Feb 15;188:112016. doi: 10.1016/j.ejmech.2019.112016. Epub 2019 Dec 28. Eur J Med Chem. 2020. PMID: 31926469 Review.
-
Recent Update on Human Lactate Dehydrogenase Enzyme 5 (hLDH5) Inhibitors: A Promising Approach for Cancer Chemotherapy.J Med Chem. 2016 Jan 28;59(2):487-96. doi: 10.1021/acs.jmedchem.5b00168. Epub 2015 Sep 4. J Med Chem. 2016. PMID: 26340601 Review.
Cited by
-
Establishment of A Nomogram for Predicting the Prognosis of Soft Tissue Sarcoma Based on Seven Glycolysis-Related Gene Risk Score.Front Genet. 2021 Dec 2;12:675865. doi: 10.3389/fgene.2021.675865. eCollection 2021. Front Genet. 2021. PMID: 34925434 Free PMC article.
-
Targeting cancer metabolism in the era of precision oncology.Nat Rev Drug Discov. 2022 Feb;21(2):141-162. doi: 10.1038/s41573-021-00339-6. Epub 2021 Dec 3. Nat Rev Drug Discov. 2022. PMID: 34862480 Free PMC article. Review.
-
5-ALA Is a Potent Lactate Dehydrogenase Inhibitor but Not a Substrate: Implications for Cell Glycolysis and New Avenues in 5-ALA-Mediated Anticancer Action.Cancers (Basel). 2022 Aug 18;14(16):4003. doi: 10.3390/cancers14164003. Cancers (Basel). 2022. PMID: 36010996 Free PMC article.
-
Tumor Microenvironment-Derived Metabolites: A Guide to Find New Metabolic Therapeutic Targets and Biomarkers.Cancers (Basel). 2021 Jun 28;13(13):3230. doi: 10.3390/cancers13133230. Cancers (Basel). 2021. PMID: 34203535 Free PMC article. Review.
-
Transcriptome-wide analysis of the differences between MCF7 cells cultured in DMEM or αMEM.PLoS One. 2024 Mar 28;19(3):e0298262. doi: 10.1371/journal.pone.0298262. eCollection 2024. PLoS One. 2024. PMID: 38547234 Free PMC article.
References
-
- Warburg O, Posener K, Negelein E. Ueber den stoffwechsel der tumoren. Biochem Z. 1924;152:319–344.
-
- Rong Y, Wu W, Ni X, Kuang T, Jin D, Wang D, Lou W. Lactate dehydrogenase A is overexpressed in pancreatic cancer and promotes the growth of pancreatic cancer cells. Tumour Biol. 2013;34:1523–1530. - PubMed
-
- Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. - PubMed
- Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–2042. - PMC - PubMed
- Sheng SL, Liu JJ, Dai YH, Sun XG, Xiong XP, Huang G. Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J. 2012;279:3898–3910. - PubMed
- Wang ZY, Loo TY, Shen JG, Wang N, Wang DM, Yang DP, Mo SL, Guan XY, Chen JP. LDH-A silencing suppresses breast cancer tumorigenicity through induction of oxidative stress mediated mitochondrial pathway apoptosis. Breast Cancer Res Treat. 2012;131:791–800. - PubMed
- Yao F, Zhao T, Zhong C, Zhu J, Zhao H. LDHA is necessary for the tumorigenicity of esophageal squamous cell carcinoma. Tumour Biol. 2012;34:25–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

