Segmented Filamentous Bacteria Provoke Lung Autoimmunity by Inducing Gut-Lung Axis Th17 Cells Expressing Dual TCRs
- PMID: 29120746
- PMCID: PMC5749641
- DOI: 10.1016/j.chom.2017.10.007
Segmented Filamentous Bacteria Provoke Lung Autoimmunity by Inducing Gut-Lung Axis Th17 Cells Expressing Dual TCRs
Abstract
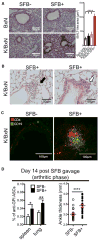
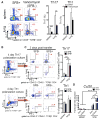
Lung complications are a major cause of rheumatoid arthritis-related mortality. Involvement of gut microbiota in lung diseases by the gut-lung axis has been widely observed, but the underlying mechanism remains mostly unknown. Using an autoimmune arthritis model, we show that a constituent of the gut microbiota, segmented filamentous bacteria (SFB), distantly provoke lung pathology. SFB induce autoantibodies in lung during the pre-arthritic phase, and SFB-dependent lung pathology requires the T helper 17 (Th17) responses. SFB-induced gut Th17 cells are preferentially recruited to lung over spleen due to robust expression in the lung of the Th17 chemoattractant, CCL20. Additionally, we found that in peripheral tissues, SFB selectively expand dual T cell receptor (TCR)-expressing Th17 cells recognizing both an SFB epitope and self-antigen, thus augmenting autoimmunity. This study reveals mechanisms for commensal-mediated gut-lung crosstalk and dual TCR-based autoimmunity.
Keywords: Th17 cells; autoimmune; dual TCR; gut microbiota; gut-lung axis; rheumatoid arthritis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


References
-
- Alam SM, Gascoigne NR. Posttranslational regulation of TCR Valpha allelic exclusion during T cell differentiation. J Immunol. 1998;160:3883–3890. - PubMed
-
- Albert LJ, Inman RD. Molecular mimicry and autoimmunity. N Engl J Med. 1999;341:2068–2074. - PubMed
-
- Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15:55–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

