Pleckstrin homology-like domain family A, member 3 (PHLDA3) deficiency improves islets engraftment through the suppression of hypoxic damage
- PMID: 29121094
- PMCID: PMC5679611
- DOI: 10.1371/journal.pone.0187927
Pleckstrin homology-like domain family A, member 3 (PHLDA3) deficiency improves islets engraftment through the suppression of hypoxic damage
Abstract
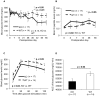
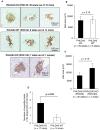
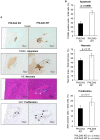
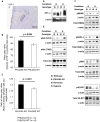
Islet transplantation is a useful cell replacement therapy that can restore the glycometabolic function of severe diabetic patients. It is known that many transplanted islets failed to engraft, and thus, new approaches for overcoming graft loss that may improve the outcome of future clinical islet transplantations are necessary. Pleckstrin homology-like domain family A, member 3 (PHLDA3) is a known suppressor of neuroendocrine tumorigenicity, yet deficiency of this gene increases islet proliferation, prevents islet apoptosis, and improves their insulin-releasing function without causing tumors. In this study, we examined the potential use of PHLDA3-deficient islets in transplantation. We observed that: 1) transplanting PHLDA3-deficient islets into diabetic mice significantly improved their glycometabolic condition, 2) the improved engraftment of PHLDA3-deficient islets resulted from increased cell survival during early transplantation, and 3) Akt activity was elevated in PHLDA3-deficient islets, especially under hypoxic conditions. Thus, we determined that PHLDA3-deficient islets are more resistant against stresses induced by islet isolation and transplantation. We conclude that use of islets with suppressed PHLDA3 expression could be a novel and promising treatment for improving engraftment and consequent glycemic control in islet transplantation.
Conflict of interest statement
Figures

Similar articles
-
p53-PHLDA3-Akt Network: The Key Regulators of Neuroendocrine Tumorigenesis.Int J Mol Sci. 2020 Jun 8;21(11):4098. doi: 10.3390/ijms21114098. Int J Mol Sci. 2020. PMID: 32521808 Free PMC article. Review.
-
Targeting uncoupling protein-2 improves islet graft function.Cell Transplant. 2011;20(3):421-9. doi: 10.3727/096368910X522243. Epub 2010 Aug 18. Cell Transplant. 2011. PMID: 20719094
-
A preconditioning regimen with a PKCɛ activator improves islet graft function in a mouse transplant model.Cell Transplant. 2014;23(7):913-9. doi: 10.3727/096368913X665567. Epub 2013 Apr 2. Cell Transplant. 2014. PMID: 23562311
-
A preexistent hypoxic gene signature predicts impaired islet graft function and glucose homeostasis.Cell Transplant. 2013;22(11):2147-59. doi: 10.3727/096368912X658728. Epub 2012 Oct 31. Cell Transplant. 2013. PMID: 23127310
-
Improving islet transplantation by gene delivery of hepatocyte growth factor (HGF) and its downstream target, protein kinase B (PKB)/Akt.Cell Biochem Biophys. 2007;48(2-3):191-9. doi: 10.1007/s12013-007-0024-7. Cell Biochem Biophys. 2007. PMID: 17709889 Review.
Cited by
-
PHLDA3 inhibition attenuates endoplasmic reticulum stress-induced apoptosis in myocardial hypoxia/reoxygenation injury by activating the PI3K/AKT signaling pathway.Exp Ther Med. 2021 Jun;21(6):613. doi: 10.3892/etm.2021.10045. Epub 2021 Apr 14. Exp Ther Med. 2021. PMID: 33936270 Free PMC article.
-
The Role of PHLDA3 in Cancer Progression and Its Potential as a Therapeutic Target.Cancers (Basel). 2025 Mar 22;17(7):1069. doi: 10.3390/cancers17071069. Cancers (Basel). 2025. PMID: 40227573 Free PMC article. Review.
-
Metabolic intervention by low carbohydrate diet suppresses the onset and progression of neuroendocrine tumors.Cell Death Dis. 2023 Sep 7;14(9):597. doi: 10.1038/s41419-023-06123-1. Cell Death Dis. 2023. PMID: 37679316 Free PMC article.
-
p53-PHLDA3-Akt Network: The Key Regulators of Neuroendocrine Tumorigenesis.Int J Mol Sci. 2020 Jun 8;21(11):4098. doi: 10.3390/ijms21114098. Int J Mol Sci. 2020. PMID: 32521808 Free PMC article. Review.
-
TSSC3 promotes autophagy via inactivating the Src-mediated PI3K/Akt/mTOR pathway to suppress tumorigenesis and metastasis in osteosarcoma, and predicts a favorable prognosis.J Exp Clin Cancer Res. 2018 Aug 9;37(1):188. doi: 10.1186/s13046-018-0856-6. J Exp Clin Cancer Res. 2018. PMID: 30092789 Free PMC article.
References
-
- Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant. 2008;8(10):1990–7. doi: 10.1111/j.1600-6143.2008.02353.x . - DOI - PubMed
-
- Fiorina P, Folli F, Bertuzzi F, Maffi P, Finzi G, Venturini M, et al. Long-term beneficial effect of islet transplantation on diabetic macro-/microangiopathy in type 1 diabetic kidney-transplanted patients. Diabetes Care. 2003;26(4):1129–36. . - PubMed
-
- Fiorina P, Folli F, Zerbini G, Maffi P, Gremizzi C, Di Carlo V, et al. Islet transplantation is associated with improvement of renal function among uremic patients with type I diabetes mellitus and kidney transplants. J Am Soc Nephrol. 2003;14(8):2150–8. . - PubMed
-
- Bassi R, Fiorina P. Impact of islet transplantation on diabetes complications and quality of life. Curr Diab Rep. 2011;11(5):355–63. doi: 10.1007/s11892-011-0211-1 . - DOI - PubMed
-
- Sakata N, Gu Y, Qi M, Yamamoto C, Hiura A, Sumi S, et al. Effect of rat-to-mouse bioartificial pancreas xenotransplantation on diabetic renal damage and survival. Pancreas. 2006;32(3):249–57. doi: 10.1097/01.mpa.0000203959.31877.8c . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

