Durability of immunity by hepatitis B vaccine in Japanese health care workers depends on primary response titers and durations
- PMID: 29121107
- PMCID: PMC5679562
- DOI: 10.1371/journal.pone.0187661
Durability of immunity by hepatitis B vaccine in Japanese health care workers depends on primary response titers and durations
Abstract
Background: Health care workers (HCWs) are frequently exposed to hepatitis B virus (HBV) infection. The efficacy and safety of immunization with the hepatitis B (HB) vaccine are well recognized, but the durability of immunity and need for booster doses in those with secondary vaccine response failure remains controversial.
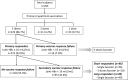
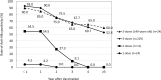
Methods: This was a retrospective cohort study performed at Osaka University Hospital, Japan. We examined antibodies against HB surface antigen (anti-HBs) titers annually after immunization for previously non-immunized HCWs. Primary responders were categorized by their sero-positive durations as short responders (those whose anti-HBs titers declined to negative range within 3 years), and long responders (those who retained positive anti-HBs levels for 3 years and more). We re-immunized short responders with either single or 3-dose boosters, the long responders with a single booster when their titers dropped below protective levels, and examined their sero-protection rates over time thereafter.
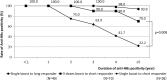
Results: From 2001 to 2012, data of 264 HCWs with a median age of 25.3 were collected. The rate of anti-HBs positivity after primary vaccination were 93.0% after three doses (n = 229), 54.5% after two doses (n = 11), and 4.2% after a single dose (n = 24). Of 213 primary responders, the anti-HBs levels of 95 participants (44.6%) fell below the protective levels, including 46 short responders and 49 long responders. HCWs with higher initial anti-HBs titers after primary vaccination had significantly longer durations of sero-positivity. For short responders, 3-dose booster vaccination induced a longer duration of anti-HBs positivity compared to a single-dose booster, whereas for long responders, a single-dose booster alone could induce prolonged anti-HBs positivity.
Conclusion: Our preliminary data suggested that it may be useful to differentiate HB vaccine responders based on their primary response durations to maintain protective levels of anti-HBs efficiently. A randomized, prospective, large-scale study is warranted to support our findings.
Conflict of interest statement
Figures

Similar articles
-
Durability of antibody response against hepatitis B virus in healthcare workers vaccinated as adults.Clin Infect Dis. 2015 Feb 15;60(4):505-13. doi: 10.1093/cid/ciu867. Epub 2014 Nov 10. Clin Infect Dis. 2015. PMID: 25389254 Free PMC article.
-
Acquisition rate of antibody to hepatitis B surface antigen among medical and dental students in Japan after three-dose hepatitis B vaccination.Vaccine. 2019 Jan 3;37(1):145-151. doi: 10.1016/j.vaccine.2018.11.019. Epub 2018 Nov 16. Vaccine. 2019. PMID: 30449632
-
Low levels of awareness, vaccine coverage, and the need for boosters among health care workers in tertiary care hospitals in India.J Gastroenterol Hepatol. 2008 Nov;23(11):1710-5. doi: 10.1111/j.1440-1746.2008.05483.x. Epub 2008 Aug 28. J Gastroenterol Hepatol. 2008. PMID: 18761556
-
Factors influencing the durability of hepatitis B vaccine responses.Vaccine. 2021 Aug 23;39(36):5224-5230. doi: 10.1016/j.vaccine.2021.07.017. Epub 2021 Jul 31. Vaccine. 2021. PMID: 34340855 Review.
-
Discordance of hepatitis B vaccination policies for healthcare workers between the USA, the UK, and Germany.Hepatol Res. 2020 Mar;50(3):272-282. doi: 10.1111/hepr.13470. Epub 2020 Jan 21. Hepatol Res. 2020. PMID: 31845478 Review.
Cited by
-
Characterization of the TCR β Chain Repertoire in Peripheral Blood from Hepatitis B Vaccine Responders and Non-Responders.J Inflamm Res. 2022 Feb 14;15:939-951. doi: 10.2147/JIR.S347702. eCollection 2022. J Inflamm Res. 2022. PMID: 35210805 Free PMC article.
-
Prevalence, Molecular Characterization, and Immune Status Response of Hepatitis B Virus Among Healthcare Workers in Yaoundé General Hospital, Cameroon, During May to June 2024: A Hospital-Based Cross-Sectional Study.Health Sci Rep. 2025 Jun 13;8(6):e70913. doi: 10.1002/hsr2.70913. eCollection 2025 Jun. Health Sci Rep. 2025. PMID: 40519605 Free PMC article.
-
Next-generation sequencing analysis of the human T-cell and B-cell receptor repertoire diversity before and after hepatitis B vaccination.Hum Vaccin Immunother. 2019;15(11):2738-2753. doi: 10.1080/21645515.2019.1600987. Epub 2019 Jul 25. Hum Vaccin Immunother. 2019. PMID: 30945971 Free PMC article.
-
Hepatitis B virus vaccination post serological testing and antibody levels of vaccinated health care workers in Accra, Ghana.Vaccine X. 2023 Apr 3;14:100294. doi: 10.1016/j.jvacx.2023.100294. eCollection 2023 Aug. Vaccine X. 2023. PMID: 37101844 Free PMC article.
-
Early-stage antibody kinetics after the third dose of BNT162b2 mRNA COVID-19 vaccination measured by a point-of-care fingertip whole blood testing.Sci Rep. 2022 Nov 30;12(1):20628. doi: 10.1038/s41598-022-24464-3. Sci Rep. 2022. PMID: 36450786 Free PMC article.
References
-
- Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8 - DOI - PubMed
-
- Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X - DOI - PubMed
-
- Bond W, Favero M, Petersen N, Gravelle C, Ebert J, Maynard J. Survival of hepatitis B virus after drying and storage for one week. Lancet. 1981;317:550–551. - PubMed
-
- Meireles LC, Marinho RT, Van Damme P. Three decades of hepatitis B control with vaccination. World J Hepatol. 2015;7:2127 doi: 10.4254/wjh.v7.i18.2127 - DOI - PMC - PubMed
-
- Jack A, Hall A, Maine N, Mendy M, Whittle H. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179:489–492. doi: 10.1086/314578 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

