Out-of-Pocket and Health Care Spending Changes for Patients Using Orally Administered Anticancer Therapy After Adoption of State Parity Laws
- PMID: 29121177
- PMCID: PMC6054307
- DOI: 10.1001/jamaoncol.2017.3598
Out-of-Pocket and Health Care Spending Changes for Patients Using Orally Administered Anticancer Therapy After Adoption of State Parity Laws
Abstract
Importance: Oral anticancer medications are increasingly important but costly treatment options for patients with cancer. By early 2017, 43 states and Washington, DC, had passed laws to ensure patients with private insurance enrolled in fully insured health plans pay no more for anticancer medications administered by mouth than anticancer medications administered by infusion. Federal legislation regarding this issue is currently pending. Despite their rapid acceptance, the changes associated with state adoption of oral chemotherapy parity laws have not been described.
Objective: To estimate changes in oral anticancer medication use, out-of-pocket spending, and health plan spending associated with oral chemotherapy parity law adoption.
Design, setting, and participants: Analysis of administrative health plan claims data from 2008-2012 for 3 large nationwide insurers aggregated by the Health Care Cost Institute. Data analysis was first completed in 2015 and updated in 2017. The study population included 63 780 adults living in 1 of 16 states that passed parity laws during the study period and who received anticancer drug treatment for which orally administered treatment options were available. Study analysis used a difference-in-differences approach.
Exposures: Time period before and after adoption of state parity laws, controlling for whether the patient was enrolled in a plan subject to parity (fully insured) or not (self-funded, exempt via the Employee Retirement Income Security Act).
Main outcomes and measures: Oral anticancer medication use, out-of-pocket spending, and total health care spending.
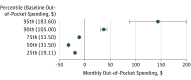
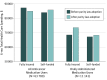
Results: Of the 63 780 adults aged 18 through 64 years, 51.4% participated in fully insured plans and 48.6% in self-funded plans (57.2% were women; 76.8% were aged 45 to 64 years). The use of oral anticancer medication treatment as a proportion of all anticancer treatment increased from 18% to 22% (adjusted difference-in-differences risk ratio [aDDRR], 1.04; 95% CI, 0.96-1.13; P = .34) comparing months before vs after parity. In plans subject to parity laws, the proportion of prescription fills for orally administered therapy without copayment increased from 15.0% to 53.0%, more than double the increase (12.3%-18.0%) in plans not subject to parity (P < .001). The proportion of patients with out-of-pocket spending of more than $100 per month increased from 8.4% to 11.1% compared with a slight decline from 12.0% to 11.7% in plans not subject to parity (P = .004). In plans subject to parity laws, estimated monthly out-of-pocket spending decreased by $19.44 at the 25th percentile, by $32.13 at the 50th percentile, and by $10.83 at the 75th percentile but increased at the 90th ($37.19) and 95th ($143.25) percentiles after parity (all P < .001, controlling for changes in plans not subject to parity). Parity laws did not increase 6-month total spending for users of any anticancer therapy or for users of oral anticancer therapy alone.
Conclusions and relevance: While oral chemotherapy parity laws modestly improved financial protection for many patients without increasing total health care spending, these laws alone may be insufficient to ensure that patients are protected from high out-of-pocket medication costs.
Conflict of interest statement
Figures
References
-
- Bach PB. Monthly and median costs of cancer drugs at the time of FDA approval 1965-2016. https://www.mskcc.org/research-areas/programs-centers/health-policy-outc.... Accessed October 2, 2017. - PubMed
-
- Dusetzina SB. Drug pricing trends for orally administered anticancer medications reimbursed by commercial health plans, 2000-2014. JAMA Oncol. 2016;2(7):-. - PubMed
-
- Andrews M. Some states mandate better coverage of oral cancer drugs. http://www.kaiserhealthnews.org/features/insuring-your-health/2012/cance.... Accessed September 18, 2013.
-
- Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32(4):306-311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

