Primary vs Secondary Endoscopic Dacryocystorhinostomy for Acute Dacryocystitis With Lacrimal Sac Abscess Formation: A Randomized Clinical Trial
- PMID: 29121183
- PMCID: PMC6583760
- DOI: 10.1001/jamaophthalmol.2017.4798
Primary vs Secondary Endoscopic Dacryocystorhinostomy for Acute Dacryocystitis With Lacrimal Sac Abscess Formation: A Randomized Clinical Trial
Abstract
Importance: Endoscopic dacryocystorhinostomy (EN-DCR) is emerging as the preferred procedure in the management of nasolacrimal duct obstructions. However, its safety and long-term efficacy in the setting of acute dacryocystitis with lacrimal sac abscess have not been well studied.
Objective: To compare outcomes of EN-DCR as primary treatment with EN-DCR as a secondary treatment after percutaneous drainage of lacrimal sac abscess in acute dacryocystitis.
Design, setting, and participants: This randomized clinical trial was conducted from October 1, 2012, to October 31, 2015, at a tertiary ophthalmic center. The assessors of success at postoperative year 1 were masked to the procedures received by the participants. All surgical procedures were performed by 2 oculoplastic surgeons with different levels of EN-DCR experience. Eligible participants had acute dacryocystitis and lacrimal sac abscess presenting within 2 weeks of onset, who were 18 to 90 years of age. Analysis was of the intention-to-treat population.
Interventions: Patients were allocated by block randomization to receive either percutaneous drainage of lacrimal sac abscess followed by EN-DCR after the acute episode subsided (control group) or primary EN-DCR within 2 weeks of presentation (intervention group). Both groups received a course of empirical systemic antibiotics (amoxicillin and clavulanic acid, 375 mg, to be taken 3 times a day for 1 week).
Main outcomes and measures: Primary outcomes were time from presentation to documentation of symptom resolution and recurrence within 3 months.
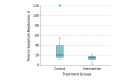
Results: Thirty-two patients were randomized equally into 2 treatment arms (control and intervention). The mean (SD) age of patients was 61 (13) years, and there was a predominance of women (27 [84%]). The mean (SD) time to symptom resolution was 13.8 (5.8) days in the intervention group compared with 31.7 (27.1) days in the control group (mean difference, 17.9; 95% CI, 3.71-32.01; P = .02). The mean (SD) time to surgery in the intervention group was shorter at 11.9 (6.3) days compared with 45.6 (30.1) days in the control group (mean difference, 33.6; 95% CI, 17.92-49.33; P < .001). Recurrences occurred once in the control group and did not occur in the intervention group. No differences in operation time and complications between the 2 groups were identified. The anatomical and functional success was 87.5% (14 of 16 cases) in both groups at postoperative year 1.
Conclusions and relevance: Primary EN-DCR in acute dacryocystitis with lacrimal sac abscess results in faster resolution compared with secondary treatment. No differences in recurrence, safety, or outcomes at postoperative year 1 were noted between the 2 treatment groups.
Conflict of interest statement
Figures
References
-
- Bharathi MJ, Ramakrishnan R, Maneksha V, Shivakumar C, Nithya V, Mittal S. Comparative bacteriology of acute and chronic dacryocystitis. Eye (Lond). 2008;22(7):953-960. - PubMed
-
- Mills DM, Bodman MG, Meyer DR, Morton AD III; ASOPRS Dacryocystitis Study Group . The microbiologic spectrum of dacryocystitis: a national study of acute versus chronic infection. Ophthal Plast Reconstr Surg. 2007;23(4):302-306. - PubMed
-
- Cahill KV, Burns JA. Management of acute dacryocystitis in adults. Ophthal Plast Reconstr Surg. 1993;9(1):38-41. - PubMed
-
- Burns JA, Cahill KV. Modified Kinosian dacryocystorhinostomy: a review of 122 cases. Ophthalmic Surg. 1985;16(11):710-716. - PubMed
-
- Ali MJ, Joshi SD, Naik MN, Honavar SG. Clinical profile and management outcome of acute dacryocystitis: two decades of experience in a tertiary eye care center. Semin Ophthalmol. 2015;30(2):118-123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

