Neurological effects of proprotein convertase subtilisin/kexin type 9 inhibitors: direct comparisons
- PMID: 29121222
- PMCID: PMC5884103
- DOI: 10.1093/ehjqcco/qcx037
Neurological effects of proprotein convertase subtilisin/kexin type 9 inhibitors: direct comparisons
Abstract
Aims: Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors considerably alter the lipid profile. We sought to examine the rates of ischaemic stroke and neurocognitive deficits in patients treated with and without PCSK9 inhibitors.
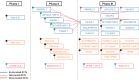
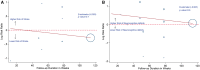
Methods and results: Randomized controlled trials (RCTs) reporting rates of ischaemic stroke and neurocognitive deficits in patients using PCSK9 inhibitors were identified. Standard meta-analysis techniques were used to compare these outcomes among patients treated with and without PCSK9 inhibitors and the two US Food and Drug Administration-approved PCSK9 inhibitors, evolocumab and alirocumab. The results were presented in terms of risk ratio (RR) with 95% confidence intervals (CIs). Sixteen RCTs with 39 104 patients were included. Evolocumab was used in six RCTs with 33 450 patients, whereas alirocumab was used in 10 RCTs with 5654 patients. We observed a significantly lower risk of ischaemic stroke among those treated with PCSK9 inhibitors (RR 0.77, 95% CI 0.64-0.93) when compared with those without. We did not observe any difference in the risk of neurocognitive deficits between the aforementioned groups (RR 1.11, 95% CI 0.93-1.32). The lower stroke risk in the PCSK9 inhibitors group was driven by evolocumab studies. We observed no difference in the risk of neurocognitive deficits among evolocumab and alirocumab when compared with no PCSK9 inhibitors group.
Conclusion: Treatment with PCSK9 inhibitors significantly lowers the risk of ischaemic stroke, without any increased risk of neurocognitive deficits. PCSK9 inhibitors are neuroprotective due to the decrease in ischaemic-mediated neurovascular events and should be considered cognitively innocuous medications.
Figures






References
-
- FDA approves Repatha to treat certain patients with high cholesterol. U.S. Food and Drug Administration. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm460082.htm (27 March 2017).
-
- FDA approves Praluent to treat certain patients with high cholesterol. U.S. Food and Drug Administration. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm455883.htm (27 March 2017).
-
- Everett BM, Smith RJ, Hiatt WR.. Reducing LDL with PCSK9 inhibitors—the clinical benefit of lipid drugs. N Engl J Med 2015;373:1588–1591. - PubMed
-
- Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, Hovingh GK, Cariou B, Gouni-Berthold I, Somaratne R, Bridges I, Scott R, Wasserman SM, Gaudet D.. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2015;385:331–340. - PubMed
-
- Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ.. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489–1499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

