Quality of life in the GLARIUS trial randomizing bevacizumab/irinotecan versus temozolomide in newly diagnosed, MGMT-nonmethylated glioblastoma
- PMID: 29121274
- PMCID: PMC6007398
- DOI: 10.1093/neuonc/nox204
Quality of life in the GLARIUS trial randomizing bevacizumab/irinotecan versus temozolomide in newly diagnosed, MGMT-nonmethylated glioblastoma
Abstract
Background: The GLARIUS trial, which investigated the efficacy of bevacizumab (BEV)/irinotecan (IRI) compared with standard temozolomide in the first-line therapy of O6-methylguanine-DNA methyltransferase (MGMT)-nonmethylated glioblastoma, showed that progression-free survival was significantly prolonged by BEV/IRI, while overall survival was similar in both arms. The present report focuses on quality of life (QoL) and Karnofsky performance score (KPS) during the whole course of the disease.
Methods: Patients (n = 170) received standard radiotherapy and were randomized (2:1) for BEV/IRI or standard temozolomide. At least every 3 months KPS was determined and QoL was measured using the European Organisation for Research and Treatment of Cancer 30-item Core Quality of Life and 20-item Brain Neoplasm questionnaires. A generalized estimating equation (GEE) model evaluated differences in the course of QoL and KPS over time. Also, the time to first deterioration and the time to postprogression deterioration were analyzed separately.
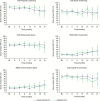
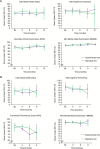
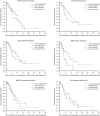
Results: In all dimensions of QoL and KPS, GEE analyses and time to first deterioration analyses did not detect significant differences between the treatment arms. At progression, 82% of patients receiving second-line therapy in the standard arm received BEV second-line therapy. For the dimensions of motor dysfunction and headaches, time to postprogression deterioration was prolonged in the standard arm receiving crossover second-line BEV in the vast majority of patients at the time of evaluation.
Conclusions: GLARIUS did not find indications for a BEV-induced detrimental effect on QoL in first-line therapy of MGMT-nonmethylated GBM patients. Moreover, GLARIUS provided some indirect corroborative data supporting the notion that BEV may have beneficial effects upon QoL in relapsed GBM.
Trial registration: ClinicalTrials.gov NCT00967330.
Figures

References
-
- Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. - PubMed
-
- Herrlinger U, Schäfer N, Steinbach JP, et al. Bevacizumab plus irinotecan versus temozolomide in newly diagnosed O6-methylguanine-DNA methyltransferase nonmethylated glioblastoma: the randomized GLARIUS Trial. J Clin Oncol. 2016;34(14):1611–1619. - PubMed
-
- Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. - PubMed
-
- Taphoorn MJ, Henriksson R, Bottomley A, et al. Health-related quality of life in a randomized phase III study of bevacizumab, temozolomide, and radiotherapy in newly diagnosed glioblastoma. J Clin Oncol. 2015;33(19):2166–2175. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

