Xanthohumol inhibits angiogenesis by suppressing nuclear factor-κB activation in pancreatic cancer
- PMID: 29121426
- PMCID: PMC5765302
- DOI: 10.1111/cas.13441
Xanthohumol inhibits angiogenesis by suppressing nuclear factor-κB activation in pancreatic cancer
Abstract
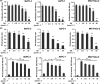
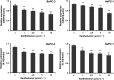
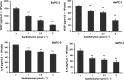
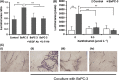
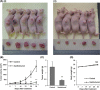
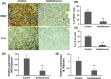
Xantohumol, a prenylated chalcone from hops (Humulus lupulus L.), has been shown to inhibit proliferation in some cancers. However, little is known regarding the effects of xanthohumol in pancreatic cancer. We have previously reported that activation of the transcription factor nuclear factor-κB (NF-κB) plays a key role in angiogenesis in pancreatic cancer. In this study, we investigated whether xanthohumol inhibited angiogenesis by blocking NF-κB activation in pancreatic cancer in vitro and in vivo. We initially confirmed that xanthohumol significantly inhibited proliferation and NF-κB activation in pancreatic cancer cell lines. Next, we demonstrated that xanthohumol significantly suppressed the expression of vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) at both the mRNA and protein levels in pancreatic cancer cell lines. We also found that coculture with BxPC-3 cells significantly enhanced tube formation in human umbilical vein endothelial cells, and treatment with xanthohumol significantly blocked this effect. In vivo, the volume of BxPC-3 subcutaneous xenograft tumors was significantly reduced in mice treated with weekly intraperitoneal injections of xanthohumol. Immunohistochemistry revealed that xanthohumol inhibited Ki-67 expression, CD31-positive microvessel density, NF-κB p65 expression, and VEGF and IL-8 levels. Taken together, these results showed, for the first time, that xanthohumol inhibited angiogenesis by suppressing NF-κB activity in pancreatic cancer. Accordingly, xanthohumol may represent a novel therapeutic agent for the management of pancreatic cancer.
Keywords: NF-κB; angiogenesis; interleukin-8; pancreatic cancer; xanthohumol.
© 2017 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures

Similar articles
-
Zerumbone inhibits angiogenesis by blocking NF-κB activity in pancreatic cancer.Pancreas. 2014 Apr;43(3):396-404. doi: 10.1097/MPA.0000000000000039. Pancreas. 2014. PMID: 24622069
-
Escin inhibits angiogenesis by suppressing interleukin‑8 and vascular endothelial growth factor production by blocking nuclear factor‑κB activation in pancreatic cancer cell lines.Oncol Rep. 2021 May;45(5):55. doi: 10.3892/or.2021.8006. Epub 2021 Mar 24. Oncol Rep. 2021. PMID: 33760162 Free PMC article.
-
The inhibitory effects of xanthohumol, a prenylated chalcone derived from hops, on cell growth and tumorigenesis in human pancreatic cancer.Biomed Pharmacother. 2015 Jul;73:40-7. doi: 10.1016/j.biopha.2015.05.020. Epub 2015 Jun 4. Biomed Pharmacother. 2015. PMID: 26211581
-
Xanthohumol: An underestimated, while potent and promising chemotherapeutic agent in cancer treatment.Prog Biophys Mol Biol. 2022 Aug;172:3-14. doi: 10.1016/j.pbiomolbio.2022.04.002. Epub 2022 Apr 8. Prog Biophys Mol Biol. 2022. PMID: 35405185 Review.
-
Xanthohumol: A Metabolite with Promising Anti-Neoplastic Potential.Anticancer Agents Med Chem. 2022;22(3):418-432. doi: 10.2174/1871520621666210223095021. Anticancer Agents Med Chem. 2022. PMID: 33622230 Review.
Cited by
-
Xanthohumol microbiome and signature in adults with Crohn's disease (the XMaS trial): a protocol for a phase II triple-masked, placebo-controlled clinical trial.Trials. 2022 Oct 22;23(1):885. doi: 10.1186/s13063-022-06782-z. Trials. 2022. PMID: 36273173 Free PMC article.
-
Inverse Molecular Docking Elucidating the Anticarcinogenic Potential of the Hop Natural Product Xanthohumol and Its Metabolites.Foods. 2022 Apr 26;11(9):1253. doi: 10.3390/foods11091253. Foods. 2022. PMID: 35563976 Free PMC article.
-
Xanthohumol Inhibits the Growth of Keratin 18-Overexpressed Esophageal Squamous Cell Carcinoma in vitro and in vivo.Front Cell Dev Biol. 2020 May 19;8:366. doi: 10.3389/fcell.2020.00366. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32509787 Free PMC article.
-
Antioxidant Potential of Xanthohumol in Disease Prevention: Evidence from Human and Animal Studies.Antioxidants (Basel). 2024 Dec 19;13(12):1559. doi: 10.3390/antiox13121559. Antioxidants (Basel). 2024. PMID: 39765887 Free PMC article. Review.
-
Xanthohumol ameliorates dextran sodium sulfate-induced colitis in mice by inhibiting of NF-κB signaling pathways and modulating intestinal microbiota.Eur J Nutr. 2024 Nov 22;64(1):21. doi: 10.1007/s00394-024-03525-5. Eur J Nutr. 2024. PMID: 39576384
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7‐30. - PubMed
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913‐2921. - PubMed
-
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first‐line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403‐2413. - PubMed
-
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817‐1825. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

