Nanomagnetic System for Rapid Diagnosis of Acute Infection
- PMID: 29121461
- PMCID: PMC6296367
- DOI: 10.1021/acsnano.7b06074
Nanomagnetic System for Rapid Diagnosis of Acute Infection
Abstract
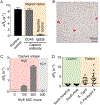
Pathogen-activated antibody-secreting cells (ASCs) produce and secrete antigen-specific antibodies. ASCs are detectable in the peripheral blood as early as 3 days after antigen exposure, which makes ASCs a potential biomarker for early disease detection. Here, we present a magnetic capture and detection (MCD) assay for sensitive, on-site detection of ASCs. In this approach, ASCs are enriched through magnetic capture, and secreted antibodies are magnetically detected by a miniaturized nuclear magnetic resonance (μNMR) system. This approach is based entirely on magnetics, which supports high contrast against biological background and simplifies assay procedures. We advanced the MCD system by (i) synthesizing magnetic nanoparticles with high magnetic moments for both cell capture and antibody detection, (ii) developing a miniaturized magnetic device for high-yield cell capture, and (iii) optimizing the μNMR assay for antibody detection. Antibody responses targeting hemolysin E (HlyE) can accurately identify individuals with acute enteric fever. As a proof-of-concept, we applied MCD to detect antibodies produced by HlyE-specific hybridoma cells. The MCD achieved high sensitivity in detecting antibodies secreted from as few as 5 hybridoma cells (50 cells/mL). Importantly, the assay could be performed with whole blood with minimal sample processing.
Keywords: acute infections; biosensors; enteric fever; host response; magnetic nanoparticles; nuclear magnetic resonance.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

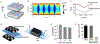
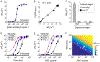

References
-
- Mogasale V; Maskery B; Ochiai RL; Lee JS; Mogasale VV; Ramani E; Kim YE; Park JK; Wierzba TF Burden of Typhoid Fever in Low-Income and Middle-Income Countries: A Systematic, Literature-Based Update with Risk-Factor Adjustment. Lancet Glob. Health 2014, 2, e570–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

