Spatiotemporal dynamics of HSV genome nuclear entry and compaction state transitions using bioorthogonal chemistry and super-resolution microscopy
- PMID: 29121649
- PMCID: PMC5697887
- DOI: 10.1371/journal.ppat.1006721
Spatiotemporal dynamics of HSV genome nuclear entry and compaction state transitions using bioorthogonal chemistry and super-resolution microscopy
Abstract
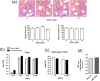
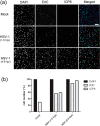
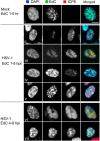
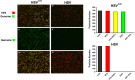
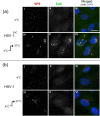
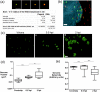
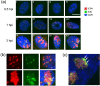
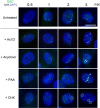
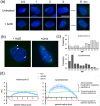
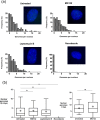
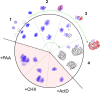
We investigated the spatiotemporal dynamics of HSV genome transport during the initiation of infection using viruses containing bioorthogonal traceable precursors incorporated into their genomes (HSVEdC). In vitro assays revealed a structural alteration in the capsid induced upon HSVEdC binding to solid supports that allowed coupling to external capture agents and demonstrated that the vast majority of individual virions contained bioorthogonally-tagged genomes. Using HSVEdC in vivo we reveal novel aspects of the kinetics, localisation, mechanistic entry requirements and morphological transitions of infecting genomes. Uncoating and nuclear import was observed within 30 min, with genomes in a defined compaction state (ca. 3-fold volume increase from capsids). Free cytosolic uncoated genomes were infrequent (7-10% of the total uncoated genomes), likely a consequence of subpopulations of cells receiving high particle numbers. Uncoated nuclear genomes underwent temporal transitions in condensation state and while ICP4 efficiently associated with condensed foci of initial infecting genomes, this relationship switched away from residual longer lived condensed foci to increasingly decondensed genomes as infection progressed. Inhibition of transcription had no effect on nuclear entry but in the absence of transcription, genomes persisted as tightly condensed foci. Ongoing transcription, in the absence of protein synthesis, revealed a distinct spatial clustering of genomes, which we have termed genome congregation, not seen with non-transcribing genomes. Genomes expanded to more decondensed forms in the absence of DNA replication indicating additional transitional steps. During full progression of infection, genomes decondensed further, with a diffuse low intensity signal dissipated within replication compartments, but frequently with tight foci remaining peripherally, representing unreplicated genomes or condensed parental strands of replicated DNA. Uncoating and nuclear entry was independent of proteasome function and resistant to inhibitors of nuclear export. Together with additional data our results reveal new insight into the spatiotemporal dynamics of HSV genome uncoating, transport and organisation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Whittaker GR, Kann M, Helenius A. Viral entry into the nucleus. Annu Rev Cell Dev Biol. 2000;16:627–51. Epub 2000/10/14. doi: 10.1146/annurev.cellbio.16.1.627 . - DOI - PubMed
-
- Greber UF, Fornerod M. Nuclear import in viral infections. Curr Top Microbiol Immunol. 2005;285:109–38. Epub 2004/12/22. . - PubMed
-
- Greber UF, Fassati A. Nuclear import of viral DNA genomes. Traffic. 2003;4(3):136–43. Epub 2003/03/27. . - PubMed
-
- Zhou L, Sokolskaja E, Jolly C, James W, Cowley SA, Fassati A. Transportin 3 promotes a nuclear maturation step required for efficient HIV-1 integration. PLoS Pathog. 2011;7(8):e1002194 Epub 2011/09/09. doi: 10.1371/journal.ppat.1002194 ; PubMed Central PMCID: PMC3161976. - DOI - PMC - PubMed
-
- Monette A, Pante N, Mouland AJ. HIV-1 remodels the nuclear pore complex. J Cell Biol. 2011;193(4):619–31. Epub 2011/05/18. doi: 10.1083/jcb.201008064 ; PubMed Central PMCID: PMC3166874. - DOI - PMC - PubMed

