Hypertrophic cardiomyopathy clinical phenotype is independent of gene mutation and mutation dosage
- PMID: 29121657
- PMCID: PMC5679632
- DOI: 10.1371/journal.pone.0187948
Hypertrophic cardiomyopathy clinical phenotype is independent of gene mutation and mutation dosage
Abstract
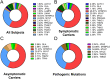
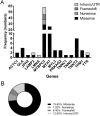
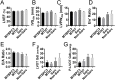
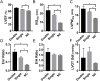
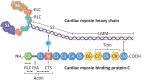
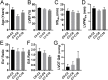
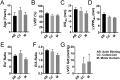
Over 1,500 gene mutations are known to cause hypertrophic cardiomyopathy (HCM). Previous studies suggest that cardiac β-myosin heavy chain (MYH7) gene mutations are commonly associated with a more severe phenotype, compared to cardiac myosin binding protein-C (MYBPC3) gene mutations with milder phenotype, incomplete penetrance and later age of onset. Compound mutations can worsen the phenotype. This study aimed to validate these comparative differences in a large cohort of individuals and families with HCM. We performed genome-phenome correlation among 80 symptomatic HCM patients, 35 asymptomatic carriers and 35 non-carriers, using an 18-gene clinical diagnostic HCM panel. A total of 125 mutations were identified in 14 genes. MYBPC3 and MYH7 mutations contributed to 50.0% and 24.4% of the HCM patients, respectively, suggesting that MYBPC3 mutations were the most frequent cause of HCM in our cohort. Double mutations were found in only nine HCM patients (7.8%) who were phenotypically indistinguishable from single-mutation carriers. Comparisons of clinical parameters of MYBPC3 and MYH7 mutants were not statistically significant, but asymptomatic carriers had high left ventricular ejection fraction and diastolic dysfunction when compared to non-carriers. The presence of double mutations increases the risk for symptomatic HCM with no change in severity, as determined in this study subset. The pathologic effects of MYBPC3 and MYH7 were found to be independent of gene mutation location. Furthermore, HCM pathology is independent of protein domain disruption in both MYBPC3 and MYH7. These data provide evidence that MYBPC3 mutations constitute the preeminent cause of HCM and that they are phenotypically indistinguishable from HCM caused by MYH7 mutations.
Conflict of interest statement
Figures

References
-
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249–54. doi: 10.1016/j.jacc.2015.01.019 . - DOI - PubMed
-
- Authors/Task Force m, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733–79. doi: 10.1093/eurheartj/ehu284 . - DOI - PubMed
-
- Varnava AM, Elliott PM, Mahon N, Davies MJ, McKenna WJ. Relation between myocyte disarray and outcome in hypertrophic cardiomyopathy. Am J Cardiol. 2001;88(3):275–9. . - PubMed
-
- O'Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J. 2014;35(30):2010–20. doi: 10.1093/eurheartj/eht439 . - DOI - PubMed
-
- Ullal AJ, Abdelfattah RS, Ashley EA, Froelicher VF. Hypertrophic Cardiomyopathy as a Cause of Sudden Cardiac Death in the Young: A Meta-Analysis. Am J Med. 2016;129(5):486–96 e2. doi: 10.1016/j.amjmed.2015.12.027 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

