Sphingolipids and Redox Signaling in Renal Regulation and Chronic Kidney Diseases
- PMID: 29121774
- PMCID: PMC5849286
- DOI: 10.1089/ars.2017.7129
Sphingolipids and Redox Signaling in Renal Regulation and Chronic Kidney Diseases
Abstract
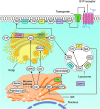
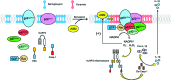
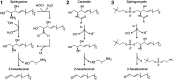
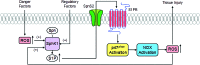
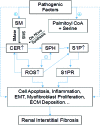
Significance: Sphingolipids play critical roles in the membrane biology and intracellular signaling events that influence cellular behavior and function. Our review focuses on the cellular mechanisms and functional relevance of the cross talk between sphingolipids and redox signaling, which may be critically implicated in the pathogenesis of different renal diseases. Recent Advances: Reactive oxygen species (ROS) and sphingolipids can regulate cellular redox homeostasis through the regulation of NADPH oxidase, mitochondrial integrity, nitric oxide synthase (NOS), and antioxidant enzymes. Over the last two decades, there have been significant advancements in the field of sphingolipid research, and it was in 2010 for the first time that sphingolipid receptor modulator was exploited as a therapeutic in humans. The cross talk of sphingolipids with redox signaling pathways becomes an important mechanism in the development of many different diseases such as renal diseases. Critical Issues: The critical issues to be addressed in this review are how sphingolipids interact with the redox signaling pathway to regulate renal function and even result in chronic kidney diseases. Ceramide, sphingosine, and sphingosine-1-phosphate (S1P) as main signaling sphingolipids are discussed in more detail. Future Directions: Although sphingolipids and ROS may mediate or modulate cellular responses to physiological and pathological stimuli, more translational studies and mechanistic pursuit in a tissue- or cell-specific way are needed to enhance our understanding of this important topic and to develop effective therapeutic strategies to treat diseases associated with redox signaling and sphingolipid cross talk. Antioxid. Redox Signal. 28, 1008-1026.
Keywords: NADPH oxidase; free radicals; inflammation; sphingolipids.
Figures



Similar articles
-
Sphingolipid signaling and redox regulation.Free Radic Biol Med. 2006 Jun 1;40(11):1875-88. doi: 10.1016/j.freeradbiomed.2006.01.035. Epub 2006 Feb 17. Free Radic Biol Med. 2006. PMID: 16716889 Review.
-
Sphingosine-1-Phosphate Metabolism and Signaling in Kidney Diseases.J Am Soc Nephrol. 2021 Jan;32(1):9-31. doi: 10.1681/ASN.2020050697. Epub 2020 Dec 18. J Am Soc Nephrol. 2021. PMID: 33376112 Free PMC article. Review.
-
The Cross-Talk Between Sphingolipids and Insulin-Like Growth Factor Signaling: Significance for Aging and Neurodegeneration.Mol Neurobiol. 2019 May;56(5):3501-3521. doi: 10.1007/s12035-018-1286-3. Epub 2018 Aug 23. Mol Neurobiol. 2019. PMID: 30140974 Free PMC article. Review.
-
Sphingolipids--the enigmatic lipid class: biochemistry, physiology, and pathophysiology.Toxicol Appl Pharmacol. 1997 Jan;142(1):208-25. doi: 10.1006/taap.1996.8029. Toxicol Appl Pharmacol. 1997. PMID: 9007051 Review.
-
The Role of Ceramide and Sphingosine-1-Phosphate in Alzheimer's Disease and Other Neurodegenerative Disorders.Mol Neurobiol. 2019 Aug;56(8):5436-5455. doi: 10.1007/s12035-018-1448-3. Epub 2019 Jan 5. Mol Neurobiol. 2019. PMID: 30612333 Free PMC article. Review.
Cited by
-
Role of sphingolipid metabolites in the homeostasis of steroid hormones and the maintenance of testicular functions.Front Endocrinol (Lausanne). 2023 Mar 17;14:1170023. doi: 10.3389/fendo.2023.1170023. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37008929 Free PMC article. Review.
-
Lipid homeostasis in diabetic kidney disease.Int J Biol Sci. 2024 Jul 2;20(10):3710-3724. doi: 10.7150/ijbs.95216. eCollection 2024. Int J Biol Sci. 2024. PMID: 39113692 Free PMC article.
-
Mitochondrial oxidative damage reprograms lipid metabolism of renal tubular epithelial cells in the diabetic kidney.Cell Mol Life Sci. 2024 Jan 11;81(1):23. doi: 10.1007/s00018-023-05078-y. Cell Mol Life Sci. 2024. PMID: 38200266 Free PMC article.
-
Medial calcification in the arterial wall of smooth muscle cell-specific Smpd1 transgenic mice: A ceramide-mediated vasculopathy.J Cell Mol Med. 2020 Jan;24(1):539-553. doi: 10.1111/jcmm.14761. Epub 2019 Nov 19. J Cell Mol Med. 2020. PMID: 31743567 Free PMC article.
-
Sex differences in obesity-induced renal lipid accumulation revealed by lipidomics: a role of adiponectin/AMPK axis.Biol Sex Differ. 2023 Sep 28;14(1):63. doi: 10.1186/s13293-023-00543-6. Biol Sex Differ. 2023. PMID: 37770988 Free PMC article.
References
-
- Achar E, Maciel TT, Collares CF, Teixeira VP, and Schor N. Amitriptyline attenuates interstitial inflammation and ameliorates the progression of renal fibrosis. Kidney Int 75: 596–604, 2009 - PubMed
-
- Ader I, Brizuela L, Bouquerel P, Malavaud B, and Cuvillier O. Sphingosine kinase 1: a new modulator of hypoxia inducible factor 1alpha during hypoxia in human cancer cells. Cancer Res 68: 8635–8642, 2008 - PubMed
-
- Alroy J, Sabnis S, and Kopp JB. Renal pathology in Fabry disease. J Am Soc Nephrol 13 Suppl 2: S134–S138, 2002 - PubMed
-
- Askari H, Kaneski CR, Semino-Mora C, Desai P, Ang A, Kleiner DE, Perlee LT, Quezado M, Spollen LE, Wustman BA, and Schiffmann R. Cellular and tissue localization of globotriaosylceramide in Fabry disease. Virchows Archiv 451: 823–834, 2007 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

