Preference option randomized design (PORD) for comparative effectiveness research: Statistical power for testing comparative effect, preference effect, selection effect, intent-to-treat effect, and overall effect
- PMID: 29121828
- PMCID: PMC6834113
- DOI: 10.1177/0962280217734584
Preference option randomized design (PORD) for comparative effectiveness research: Statistical power for testing comparative effect, preference effect, selection effect, intent-to-treat effect, and overall effect
Abstract
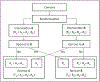
Comparative effectiveness research trials in real-world settings may require participants to choose between preferred intervention options. A randomized clinical trial with parallel experimental and control arms is straightforward and regarded as a gold standard design, but by design it forces and anticipates the participants to comply with a randomly assigned intervention regardless of their preference. Therefore, the randomized clinical trial may impose impractical limitations when planning comparative effectiveness research trials. To accommodate participants' preference if they are expressed, and to maintain randomization, we propose an alternative design that allows participants' preference after randomization, which we call a "preference option randomized design (PORD)". In contrast to other preference designs, which ask whether or not participants consent to the assigned intervention after randomization, the crucial feature of preference option randomized design is its unique informed consent process before randomization. Specifically, the preference option randomized design consent process informs participants that they can opt out and switch to the other intervention only if after randomization they actively express the desire to do so. Participants who do not independently express explicit alternate preference or assent to the randomly assigned intervention are considered to not have an alternate preference. In sum, preference option randomized design intends to maximize retention, minimize possibility of forced assignment for any participants, and to maintain randomization by allowing participants with no or equal preference to represent random assignments. This design scheme enables to define five effects that are interconnected with each other through common design parameters-comparative, preference, selection, intent-to-treat, and overall/as-treated-to collectively guide decision making between interventions. Statistical power functions for testing all these effects are derived, and simulations verified the validity of the power functions under normal and binomial distributions.
Keywords: Preference; comparative effectiveness research; decision making; power; randomization.
Conflict of interest statement
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Letter to the Editor: Preference option randomized design (PORD) for comparative effectiveness research: Statistical power for testing comparative effect, preference effect, selection effect, intent-to-treat effect, and overall effect (SMMR, Vol. 28, Issue 2, 2019).Stat Methods Med Res. 2019 May;28(5):1597-1598. doi: 10.1177/0962280218767691. Epub 2018 Apr 10. Stat Methods Med Res. 2019. PMID: 29633629 No abstract available.
-
Comment to the reply letter: Letter to the Editor: Preference option randomized design (PORD) for comparative effectiveness research: Statistical power for testing comparative effect, preference effect, selection effect, intent-to-treat effect, and overall effect (SMMR, Vol. 28, Issue 2, 2019).Stat Methods Med Res. 2019 May;28(5):1603. doi: 10.1177/0962280218768107. Epub 2018 Apr 10. Stat Methods Med Res. 2019. PMID: 29633654 No abstract available.
-
Ethical pitfalls in neonatal comparative effectiveness trials.Neonatology. 2014;105(4):350-1. doi: 10.1159/000360650. Epub 2014 May 30. Neonatology. 2014. PMID: 24931328
-
Cluster over individual randomization: are study design choices appropriately justified? Review of a random sample of trials.Clin Trials. 2020 Jun;17(3):253-263. doi: 10.1177/1740774519896799. Epub 2020 May 5. Clin Trials. 2020. PMID: 32367741 Review.
-
Proposals to Conduct Randomized Controlled Trials Without Informed Consent: a Narrative Review.J Gen Intern Med. 2016 Dec;31(12):1511-1518. doi: 10.1007/s11606-016-3780-5. Epub 2016 Jul 6. J Gen Intern Med. 2016. PMID: 27384536 Free PMC article. Review.
Cited by
-
Comparative effects of telephone versus in-office behavioral counseling to improve HIV treatment outcomes among people living with HIV in a rural setting.Transl Behav Med. 2021 Apr 7;11(3):852-862. doi: 10.1093/tbm/ibaa109. Transl Behav Med. 2021. PMID: 33200772 Free PMC article.
-
Utilizing patient perception of group treatment in exploring medication adherence, social support, and quality of life outcomes in people who inject drugs with hepatitis C.J Subst Abuse Treat. 2021 Jul;126:108459. doi: 10.1016/j.jsat.2021.108459. Epub 2021 May 7. J Subst Abuse Treat. 2021. PMID: 34116813 Free PMC article. Clinical Trial.
-
Design, analysis, and interpretation of treatment response heterogeneity in personalized nutrition and obesity treatment research.Obes Rev. 2023 Dec;24(12):e13635. doi: 10.1111/obr.13635. Epub 2023 Sep 4. Obes Rev. 2023. PMID: 37667550 Free PMC article. Review.
-
Causal models and causal modelling in obesity: foundations, methods and evidence.Philos Trans R Soc Lond B Biol Sci. 2023 Oct 23;378(1888):20220227. doi: 10.1098/rstb.2022.0227. Epub 2023 Sep 4. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37661742 Free PMC article. Review.
-
Factors associated with choice of behavioural weight loss program by adults with obesity.Clin Obes. 2023 Aug;13(4):e12591. doi: 10.1111/cob.12591. Epub 2023 Apr 11. Clin Obes. 2023. PMID: 37038768 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

