Detection of circulating miRNAs: comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients
- PMID: 29121858
- PMCID: PMC5679326
- DOI: 10.1186/s12885-017-3737-z
Detection of circulating miRNAs: comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients
Abstract
Background: Circulating cell-free miRNAs have emerged as promising minimally-invasive biomarkers for early detection, prognosis and monitoring of cancer. They can exist in the bloodstream incorporated into extracellular vesicles (EVs) and ribonucleoprotein complexes. However, it is still debated if EVs contain biologically meaningful amounts of miRNAs and may provide a better source of miRNA biomarkers than whole plasma. The aim of this study was to systematically compare the diagnostic potential of prostate cancer-associated miRNAs in whole plasma and in plasma EVs.
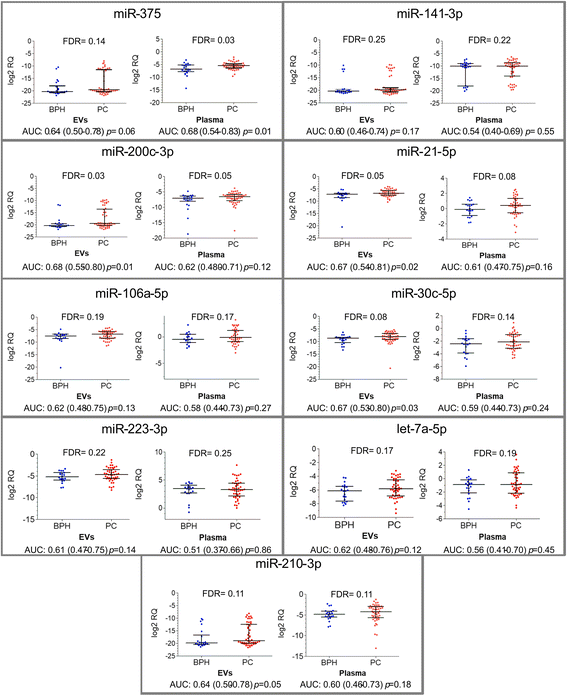
Methods: RNA was isolated from whole plasma and plasma EV samples from a well characterised cohort of 50 patient with prostate cancer (PC) and 22 patients with benign prostatic hyperplasia (BPH). Nine miRNAs known to have a diagnostic potential for PC in cell-free blood were quantified by RT-qPCR and the relative quantities were compared between patients with PC and BPH and between PC patients with Gleason score ≥ 8 and ≤6.
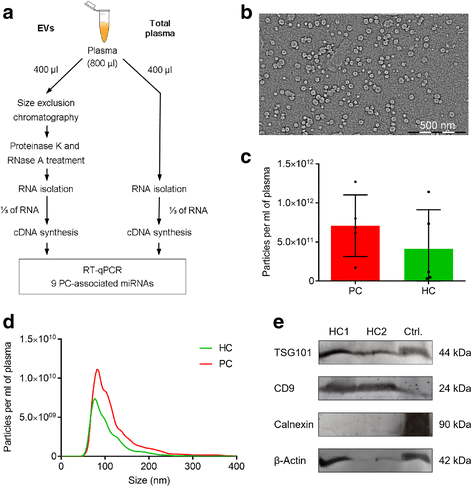
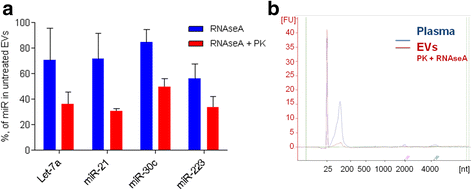
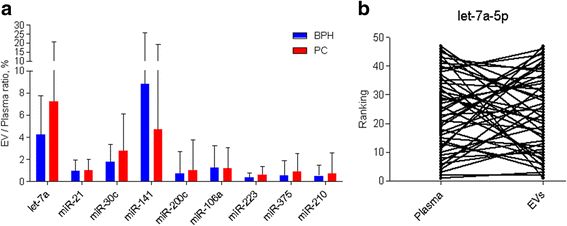
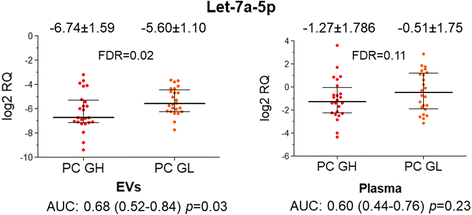
Results: Only a small fraction of the total cell-free miRNA was recovered from the plasma EVs, however the EV-incorporated and whole plasma cell-free miRNA profiles were clearly different. Four of the miRNAs analysed showed a diagnostic potential in our patient cohort. MiR-375 could differentiate between PC and BPH patients when analysed in the whole plasma, while miR-200c-3p and miR-21-5p performed better when analysed in plasma EVs. EV-incorporated but not whole plasma Let-7a-5p level could distinguish PC patients with Gleason score ≥ 8 vs ≤6.
Conclusions: This study demonstrates that for some miRNA biomarkers EVs provide a more consistent source of RNA than whole plasma, while other miRNAs show better diagnostic performance when tested in the whole plasma.
Keywords: Biomarkers; Cell-free miRNAs; Exosomes; Extracellular vesicles; Liquid biopsy; Microvesicles; Prostate cancer.
Conflict of interest statement
Ethics approval and consent to participate
Biobanking procedures were approved by the Committee of Medical Ethics of Latvia (decision No.5, 16.09.2010) and the use of clinical samples for research was approved by the Committee of Biomedical Ethics of Riga East University Hospital (decision No. 7-A/15, 04.06.2015). The blood samples were collected after the patients’ informed written consent was obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

