Multiplex real-time PCR assay for detection of Escherichia coli O157:H7 and screening for non-O157 Shiga toxin-producing E. coli
- PMID: 29121863
- PMCID: PMC5679507
- DOI: 10.1186/s12866-017-1123-2
Multiplex real-time PCR assay for detection of Escherichia coli O157:H7 and screening for non-O157 Shiga toxin-producing E. coli
Abstract
Background: Shiga toxin-producing Escherichia coli (STEC), including E. coli O157:H7, are responsible for numerous foodborne outbreaks annually worldwide. E. coli O157:H7, as well as pathogenic non-O157:H7 STECs, can cause life-threating complications, such as bloody diarrhea (hemolytic colitis) and hemolytic-uremic syndrome (HUS). Previously, we developed a real-time PCR assay to detect E. coli O157:H7 in foods by targeting a unique putative fimbriae protein Z3276. To extend the detection spectrum of the assay, we report a multiplex real-time PCR assay to specifically detect E. coli O157:H7 and screen for non-O157 STEC by targeting Z3276 and Shiga toxin genes (stx1 and stx2). Also, an internal amplification control (IAC) was incorporated into the assay to monitor the amplification efficiency.
Methods: The multiplex real-time PCR assay was developed using the Life Technology ABI 7500 System platform and the standard chemistry. The optimal amplification mixture of the assay contains 12.5 μl of 2 × Universal Master Mix (Life Technology), 200 nM forward and reverse primers, appropriate concentrations of four probes [(Z3276 (80 nM), stx1 (80 nM), stx2 (20 nM), and IAC (40 nM)], 2 μl of template DNA, and water (to make up to 25 μl in total volume). The amplification conditions of the assay were set as follows: activation of TaqMan at 95 °C for 10 min, then 40 cycles of denaturation at 95 °C for 10 s and annealing/extension at 60 °C for 60 s.
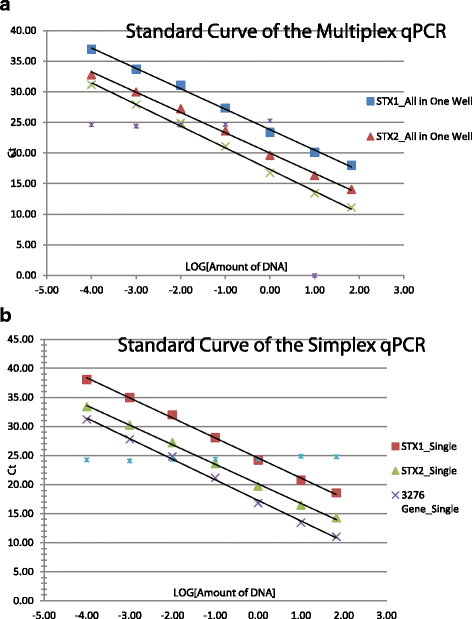
Results: The multiplex assay was optimized for amplification conditions. The limit of detection (LOD) for the multiplex assay was determined to be 200 fg of bacterial DNA, which is equivalent to 40 CFU per reaction which is similar to the LOD generated in single targeted PCRs. Inclusivity and exclusivity determinants were performed with 196 bacterial strains. All E. coli O157:H7 (n = 135) were detected as positive and all STEC strains (n = 33) were positive for stx1, or stx2, or stx1 and stx2 (Table 1). No cross reactivity was detected with Salmonella enterica, Shigella strains, or any other pathogenic strains tested.
Conclusions: A multiplex real-time PCR assay that can rapidly and simultaneously detect E. coli O157:H7 and screen for non-O157 STEC strains has been developed and assessed for efficacy. The inclusivity and exclusivity tests demonstrated high sensitivity and specificity of the multiplex real-time PCR assay. In addition, this multiplex assay was shown to be effective for the detection of E. coli O157:H7 from two common food matrices, beef and spinach, and may be applied for detection of E. coli O157:H7 and screening for non-O157 STEC strains from other food matrices as well.
Keywords: Escherichia coli O157:H7; Limit of detection (LOD); Multiplex real-time PCR; Pathogen detection; Sensitivity; Shiga toxin-producing E. col (STEC); Shiga toxins (stx1, stx2); non-O157.
Conflict of interest statement
Ethics approval and consent for participation
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Figures
References
-
- Schimmer B, Nygard K, Eriksen HM, Lassen J, Lindstedt BA, Brandal LT, Kapperud G, Aavitsland P. Outbreak of haemolytic uraemic syndrome in Norway caused by stx2-positive Escherichia coli O103:H25 traced to cured mutton sausages. BMC Infect Dis. 2008;8:41. doi: 10.1186/1471-2334-8-41. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

