Ablation of epidermal RXRα in cooperation with activated CDK4 and oncogenic NRAS generates spontaneous and acute neonatal UVB induced malignant metastatic melanomas
- PMID: 29121869
- PMCID: PMC5679438
- DOI: 10.1186/s12885-017-3714-6
Ablation of epidermal RXRα in cooperation with activated CDK4 and oncogenic NRAS generates spontaneous and acute neonatal UVB induced malignant metastatic melanomas
Abstract
Background: Understanding the underlying molecular mechanisms involved in the formation of cutaneous malignant melanoma is critical for improved diagnosis and treatment. Keratinocytic nuclear receptor Retinoid X Receptor α (RXRα) has a protective role against melanomagenesis and is involved in the regulation of keratinocyte and melanocyte homeostasis subsequent acute ultraviolet (UV) irradiation.
Methods: We generated a trigenic mouse model system (RXRα ep-/- | Tyr-NRAS Q61K | CDK4 R24C/R24C ) harboring an epidermal knockout of Retinoid X Receptor α (RXRα ep-/- ), combined with oncogenic NRAS Q61K (constitutively active RAS) and activated CDK4 R24C/R24C (constitutively active CDK4). Those mice were subjected to a single neonatal dose of UVB treatment and the role of RXR α was evaluated by characterizing the molecular and cellular changes that took place in the untreated and UVB treated trigenic RXRα ep-/- mice compared to the control mice with functional RXRα.
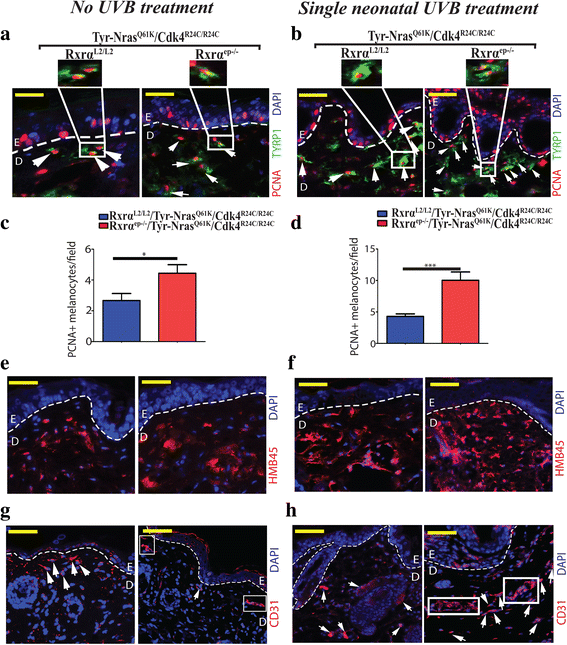
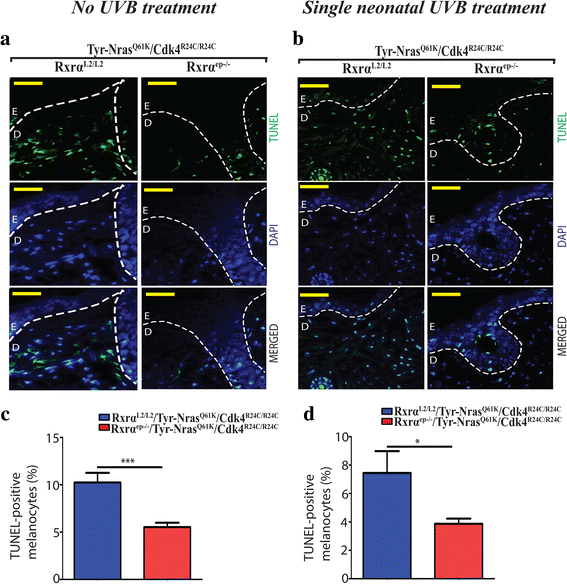
Results: Here we report that the trigenic mice develops spontaneous melanoma and exposure to a single neonatal UVB treatment reduces the tumor latency in those mice compared to control mice with functional RXRα. Melanomas from the trigenic RXRα ep-/- mice are substantial in size, show increased proliferation, exhibit increased expression of malignant melanoma markers and exhibit enhanced vascularization. Altered expression of several biomarkers including increased expression of activated AKT, p21 and cyclin D1 and reduced expression of pro-apoptotic marker BAX was observed in the tumor adjacent normal (TAN) skin of acute ultraviolet B treated trigenic RXRα ep-/- mice. Interestingly, we observed a significant increase in p21 and Cyclin D1 in the TAN skin of un-irradiated trigenic RXRα ep-/- mice, suggesting that those changes might be consequences of loss of functional RXRα in the melanoma microenvironment. Loss of RXRα in the epidermal keratinocytes in combination with oncogenic NRAS Q61K and CDK4 R24C/R24C mutations in trigenic mice led to significant melanoma invasion into the draining lymph nodes as compared to controls with functional RXRα.
Conclusions: Our study demonstrates the protective role of keratinocytic RxRα in (1) suppressing spontaneous and acute UVB-induced melanoma, and (2) preventing progression of the melanoma to malignancy in the presence of driver mutations like activated CDK4 R24C/R24C and oncogenic NRAS Q61K .
Keywords: Acute UVB; CDK4R24C/R24C; Keratinocytes; Malignant melanoma; Melanocytes; Microenvironment; NRASQ61K; Retinoid-X-receptor α (RXRα); Spontaneous melanoma; Trigenic.
Conflict of interest statement
Ethics approval
The current study was approved by the Institutional Animal Care and Use Committee (IACUC) at Oregon State University and all the animal studies are in compliance with the institutional regulations concerning the care and use of animals in the most ethical and humane way.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Loss of keratinocytic RXRα combined with activated CDK4 or oncogenic NRAS generates UVB-induced melanomas via loss of p53 and PTEN in the tumor microenvironment.Mol Cancer Res. 2015 Jan;13(1):186-96. doi: 10.1158/1541-7786.MCR-14-0164. Epub 2014 Sep 4. Mol Cancer Res. 2015. PMID: 25189354 Free PMC article.
-
Neonatal UVB exposure accelerates melanoma growth and enhances distant metastases in Hgf-Cdk4(R24C) C57BL/6 mice.Int J Cancer. 2011 Jul 15;129(2):285-94. doi: 10.1002/ijc.25913. Epub 2011 Apr 21. Int J Cancer. 2011. PMID: 21207411
-
Loss of nuclear receptor RXRα in epidermal keratinocytes promotes the formation of Cdk4-activated invasive melanomas.Pigment Cell Melanoma Res. 2010 Oct;23(5):635-48. doi: 10.1111/j.1755-148X.2010.00732.x. Epub 2010 Jul 9. Pigment Cell Melanoma Res. 2010. PMID: 20629968 Free PMC article.
-
Functional interplay between secreted ligands and receptors in melanoma.Semin Cell Dev Biol. 2018 Jun;78:73-84. doi: 10.1016/j.semcdb.2017.06.021. Epub 2017 Jul 1. Semin Cell Dev Biol. 2018. PMID: 28676423 Review.
-
UV-Induced Molecular Signaling Differences in Melanoma and Non-melanoma Skin Cancer.Adv Exp Med Biol. 2017;996:27-40. doi: 10.1007/978-3-319-56017-5_3. Adv Exp Med Biol. 2017. PMID: 29124688 Review.
Cited by
-
The Challenging Melanoma Landscape: From Early Drug Discovery to Clinical Approval.Cells. 2021 Nov 9;10(11):3088. doi: 10.3390/cells10113088. Cells. 2021. PMID: 34831311 Free PMC article. Review.
-
Conformationally Defined Rexinoids for the Prevention of Inflammation and Nonmelanoma Skin Cancers.J Med Chem. 2022 Nov 10;65(21):14409-14423. doi: 10.1021/acs.jmedchem.2c00735. Epub 2022 Nov 1. J Med Chem. 2022. PMID: 36318154 Free PMC article.
-
The Role of the Vitamin D Receptor in the Pathogenesis, Prognosis, and Treatment of Cutaneous Melanoma.Front Oncol. 2021 Oct 6;11:743667. doi: 10.3389/fonc.2021.743667. eCollection 2021. Front Oncol. 2021. PMID: 34692525 Free PMC article. Review.
-
Classification and Grading of Melanocytic Lesions in a Mouse Model of NRAS-driven Melanomagenesis.J Histochem Cytochem. 2021 Mar;69(3):203-218. doi: 10.1369/0022155420977970. Epub 2020 Dec 7. J Histochem Cytochem. 2021. PMID: 33283624 Free PMC article.
-
Crosstalk in Skin: Loss of Desmoglein 1 in Keratinocytes Inhibits BRAFV600E-Induced Cellular Senescence in Human Melanocytes.J Invest Dermatol. 2025 Jul;145(7):1740-1752.e4. doi: 10.1016/j.jid.2024.10.608. Epub 2024 Nov 23. J Invest Dermatol. 2025. PMID: 39581457
References
-
- Am Cancer Soc Cancer Facts & Figures 2016. In. Atlanta: American Cancer Society; 2016.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

