Successful production of the potato antimicrobial peptide Snakin-1 in baculovirus-infected insect cells and development of specific antibodies
- PMID: 29121909
- PMCID: PMC5679188
- DOI: 10.1186/s12896-017-0401-2
Successful production of the potato antimicrobial peptide Snakin-1 in baculovirus-infected insect cells and development of specific antibodies
Abstract
Background: Snakin-1 (StSN1) is a broad-spectrum antimicrobial cysteine-rich peptide isolated from Solanum tuberosum. Its biotechnological potential has been already recognized since it exhibits in vivo antifungal and antibacterial activity. Most attempts to produce StSN1, or homologous peptides, in a soluble native state using bacterial, yeast or synthetic expression systems have presented production bottlenecks such as insolubility, misfolding or low yields.
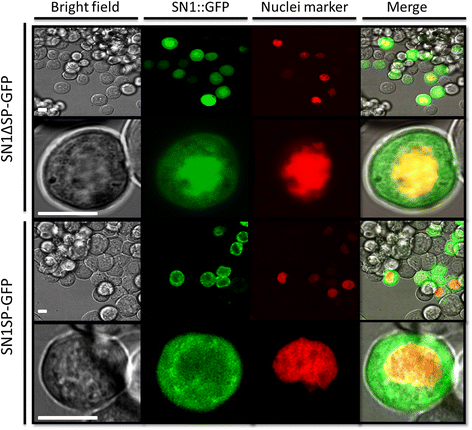
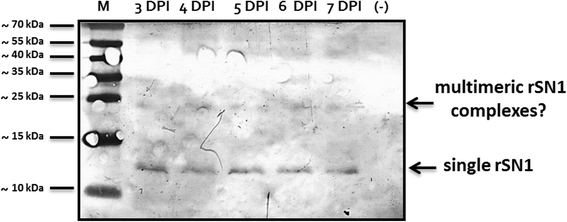
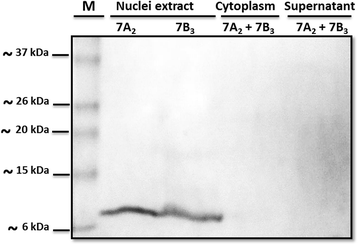
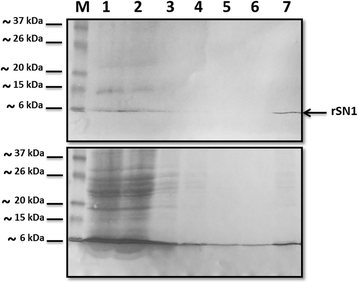
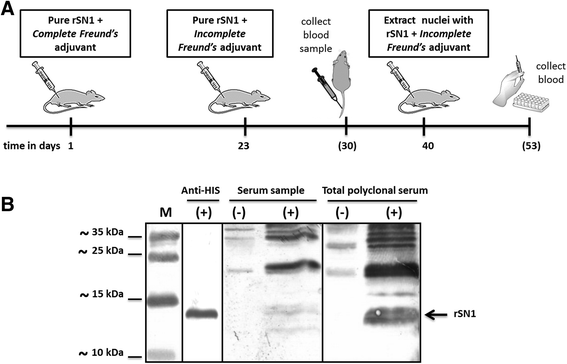
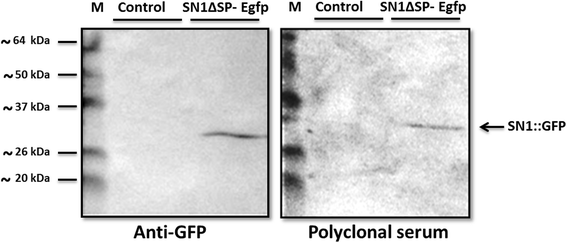
Results: In this work, we successfully expressed a recombinant StSN1 (rSN1) in Spodoptera frugiperda (Sf9) insect cells by optimizing several of the parameters for its expression in the baculovirus expression system. The recombinant peptide lacking its putative signal peptide was soluble and was present in the nuclear fraction of infected Sf9 cells. An optimized purification procedure allowed the production of rSN1 that was used for immunization of mice, which gave rise to polyclonal antibodies that detect the native protein in tissue extracts of both agroinfiltrated plants and stable transgenic lines. Our results demonstrated that this system circumvents all the difficulties associated with recombinant antimicrobial peptides expression in other heterologous systems.
Conclusions: The present study is the first report of a successful protocol to produce a soluble Snakin/GASA peptide in baculovirus-infected insect cells. Our work demonstrates that the nuclear localization of rSN1 in insect cells can be exploited for its large-scale production and subsequent generation of specific anti-rSN1 antibodies. We suggest the use of the baculovirus system for high-level expression of Snakin/GASA peptides, for biological assays, structural and functional analysis and antibody production, as an important step to both elucidate their accurate physiological role and to deepen the study of their biotechnological uses.
Keywords: Antimicrobial peptide; Baculovirus expression system; Cysteine-rich; GASA; Snakin-1; Spodoptera frugiperda Sf9 cells.
Conflict of interest statement
Ethics approval
The animal experiments were approved by our Institutional Experimentation Animal Committee (CICUAE-INTA). Animal handling and experimental procedures were strictly in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Isoflurane was used as anesthetic to collect total mice blood and then euthanize by cervical dislocation. Maximum efforts were made to minimize mice suffering.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Expression and purification of the antimicrobial peptide GSL1 in bacteria for raising antibodies.BMC Res Notes. 2014 Nov 4;7:777. doi: 10.1186/1756-0500-7-777. BMC Res Notes. 2014. PMID: 25367168 Free PMC article.
-
Expression and Purification of SH2 Domains Using Baculovirus Expression System.Methods Mol Biol. 2017;1555:183-198. doi: 10.1007/978-1-4939-6762-9_11. Methods Mol Biol. 2017. PMID: 28092034
-
Avidin is a promising tag for fusion proteins produced in baculovirus-infected insect cells.Protein Expr Purif. 1999 Oct;17(1):139-45. doi: 10.1006/prep.1999.1123. Protein Expr Purif. 1999. PMID: 10497079
-
Potato Snakin-1: an antimicrobial player of the trade-off between host defense and development.Plant Cell Rep. 2020 Jul;39(7):839-849. doi: 10.1007/s00299-020-02557-5. Epub 2020 Jun 11. Plant Cell Rep. 2020. PMID: 32529484 Review.
-
Production of recombinant androgen receptor in a heterologous expression system.Clin Chem. 1993 Feb;39(2):346-52. Clin Chem. 1993. PMID: 8432026 Review.
Cited by
-
The future of recombinant host defense peptides.Microb Cell Fact. 2022 Dec 21;21(1):267. doi: 10.1186/s12934-022-01991-2. Microb Cell Fact. 2022. PMID: 36544150 Free PMC article. Review.
-
Amino acid-derived defense metabolites from plants: A potential source to facilitate novel antimicrobial development.J Biol Chem. 2021 Jan-Jun;296:100438. doi: 10.1016/j.jbc.2021.100438. Epub 2021 Feb 19. J Biol Chem. 2021. PMID: 33610552 Free PMC article. Review.
-
StTCTP Positively Regulates StSN2 to Enhance Drought Stress Tolerance in Potato by Scavenging Reactive Oxygen Species.Int J Mol Sci. 2025 Mar 20;26(6):2796. doi: 10.3390/ijms26062796. Int J Mol Sci. 2025. PMID: 40141438 Free PMC article.
-
GASA Proteins: Review of Their Functions in Plant Environmental Stress Tolerance.Plants (Basel). 2023 May 21;12(10):2045. doi: 10.3390/plants12102045. Plants (Basel). 2023. PMID: 37653962 Free PMC article. Review.
-
Functional Analysis of Durum Wheat GASA1 Protein as a Biotechnological Alternative Against Plant Fungal Pathogens and a Positive Regulator of Biotic Stress Defense.Plants (Basel). 2025 Jan 2;14(1):112. doi: 10.3390/plants14010112. Plants (Basel). 2025. PMID: 39795373 Free PMC article.
References
-
- Terra IA, Portugal CS, Becker-Ritt AB. The battle against microbial pathogens: basic science, technological advances and educational programs. 2015. Plant antimicrobial peptides; pp. 199–207.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

