"No generics, Doctor!" The perspective of general practitioners in two French regions
- PMID: 29121918
- PMCID: PMC5680768
- DOI: 10.1186/s12913-017-2682-5
"No generics, Doctor!" The perspective of general practitioners in two French regions
Abstract
Background: Generic medicines are essential to controlling health expenditures. Their market share is still small in France. The discourse and practices of prescribers may play a major role in their use. The purpose of this study was to explore the knowledge, attitudes and practices of general practitioners (GPs) toward generic medicines in two French regions with the lowest penetration rate of these products.
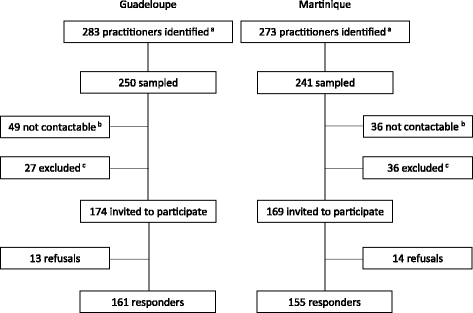
Methods: An observational study was carried out from October 2015 to February 2016 in Guadeloupe and Martinique. The first qualitative phase involved a diversified sample of 14 GPs who underwent semi-structured interviews. The second phase involved a random sample of 316 GPs (response rate = 74%) who were administered a structured questionnaire developed from the results of the first phase.
Results: Seventy-eight percent of the participants defined a generic drug as a drug containing an active substance identical to a brand-name drug, but only 11% considered generic drugs to be equivalent to brand-name drugs, and the same proportion believed that the generic drugs were of doubtful quality. The primary recognized advantage of generic medicines was their lower cost (82%). The main drawbacks cited were the variability of their presentation (44%), the confusion that they caused for some patients (47%), frequent allegations of adverse side effects (37%) and a lack of efficacy (24%), and frequent refusal by patients (26%). Seventy-four percent of the participants stated that they adapted their prescribing practices to the situation, and of this group, 47% prescribed the originator product simply on demand.
Conclusion: Most surveyed GPs were not hostile towards generic medicines. They were caught between the requirements of health insurance regimes and the opposition of numerous users and suggested that the patient information provided by health authorities should be improved and that drug composition and packaging should be made uniform.
Keywords: Drug substitution; Health behavior; Health expenditure; Primary health care.
Conflict of interest statement
Ethics approval and consent to participate
Participation in the study was voluntary, and all participants gave their informed consent. In accordance with the legislation applicable in all French regions, including Guadeloupe and Martinique, the study was registered with and approved by the National Commission for Data Protection and Liberties (CNIL) with registration number A6D1908698 M.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Beliefs, perceptions and behaviours of GPs towards generic medicines.Fam Pract. 2014 Aug;31(4):467-74. doi: 10.1093/fampra/cmu024. Epub 2014 Jun 3. Fam Pract. 2014. PMID: 24895136
-
Factors influencing the use of the "not for generic substitution" mention for prescriptions in primary care: a survey with general practitioners.BMC Health Serv Res. 2018 Nov 12;18(1):850. doi: 10.1186/s12913-018-3652-2. BMC Health Serv Res. 2018. PMID: 30419890 Free PMC article.
-
A survey exploring knowledge and perceptions of general practitioners towards the use of generic medicines in the northern state of Malaysia.Health Policy. 2010 May;95(2-3):229-35. doi: 10.1016/j.healthpol.2009.11.019. Epub 2009 Dec 30. Health Policy. 2010. PMID: 20044165
-
Generics: keep a balanced view.Prescrire Int. 2014 Feb;23(146):52-5. Prescrire Int. 2014. PMID: 24669392 Review.
-
Analysis of French generic medicines retail market: why the use of generic medicines is limited.Expert Rev Pharmacoecon Outcomes Res. 2014 Dec;14(6):795-803. doi: 10.1586/14737167.2014.946011. Epub 2014 Aug 6. Expert Rev Pharmacoecon Outcomes Res. 2014. PMID: 25095903 Review.
Cited by
-
Access to unauthorized hepatitis C generics: Perception and knowledge of physicians, pharmacists, patients and non-healthcare professionals.PLoS One. 2019 Oct 10;14(10):e0223649. doi: 10.1371/journal.pone.0223649. eCollection 2019. PLoS One. 2019. PMID: 31600328 Free PMC article.
-
Out-of-pocket payments in the context of a free maternal health care policy in Burkina Faso: a national cross-sectional survey.Health Econ Rev. 2019 Mar 27;9(1):11. doi: 10.1186/s13561-019-0228-8. Health Econ Rev. 2019. PMID: 30919219 Free PMC article.
-
Do Out-of-Pocket Payments for Care for Children under 5 Persist Even in a Context of Free Healthcare in Burkina Faso? Evidence from a Cross-Sectional Population-Based Survey.Healthcare (Basel). 2023 May 10;11(10):1379. doi: 10.3390/healthcare11101379. Healthcare (Basel). 2023. PMID: 37239664 Free PMC article.
-
A randomised survey of the quality of antibiotics and other essential medicines in Indonesia, with volume-adjusted estimates of the prevalence of substandard medicines.PLOS Glob Public Health. 2024 Dec 12;4(12):e0003999. doi: 10.1371/journal.pgph.0003999. eCollection 2024. PLOS Glob Public Health. 2024. PMID: 39666666 Free PMC article.
-
Comparison of treatment patterns, efficacy and safety between generic and branded atorvastatin users in China: a multicenter, retrospective propensity score-matched cohort study.Lipids Health Dis. 2025 Jul 22;24(1):248. doi: 10.1186/s12944-025-02673-9. Lipids Health Dis. 2025. PMID: 40696368 Free PMC article.
References
-
- Organisation for Economic Co-operation and Development. Statistics/OECD Health Statistics [Internet]. [cited 2017 Jul 28]; Available from: doi:10.1787/health-data-en
-
- OECD. Health at a Glance 2015: OECD indicators [internet]. Paris: OECD publishing; 2015 [cited 2017 Jul 27]. Available from: http://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-...
-
- IMS Institute for Healthcare Informatics. The Role of Generic Medicines in Sustaining Healthcare Systems: A European Perspective [Internet]. Parsippany, NJ 07054, USA: imshealth; 2015 [cited 2017 Jul 29]. Available from: http://www.imshealth.com/files/web/IMSH%20Institute/Healthcare%20Briefs/...
-
- World Health Organization. The WHO expert committee on specifications for pharmaceutical preparations [Internet]. Geneva: [cited 2017 Jul 29]. Available from: http://www.who.int/medicines/publications/BrochurePharmaupdatedversion.pdf
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

