Interleukin-1β signaling in fenestrated capillaries is sufficient to trigger sickness responses in mice
- PMID: 29121947
- PMCID: PMC5680784
- DOI: 10.1186/s12974-017-0990-7
Interleukin-1β signaling in fenestrated capillaries is sufficient to trigger sickness responses in mice
Abstract
Background: The physiological and behavioral symptoms of sickness, including fever, anorexia, behavioral depression, and weight loss can be both beneficial and detrimental. These sickness responses are triggered by pro-inflammatory cytokines acting on cells within the brain. Previous research demonstrates that the febrile response to peripheral insults depends upon prostaglandin production by vascular endothelial cells, but the mechanisms and specific cell type(s) responsible for other sickness responses remain unknown. The purpose of the present study was to identify which cells within the brain are required for sickness responses triggered by central nervous system inflammation.
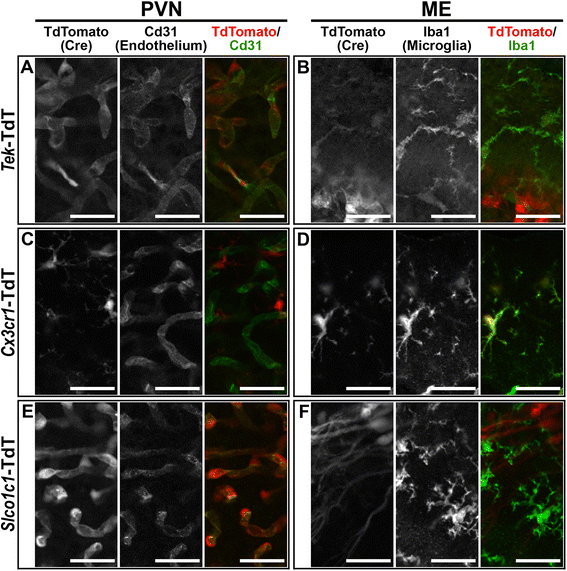
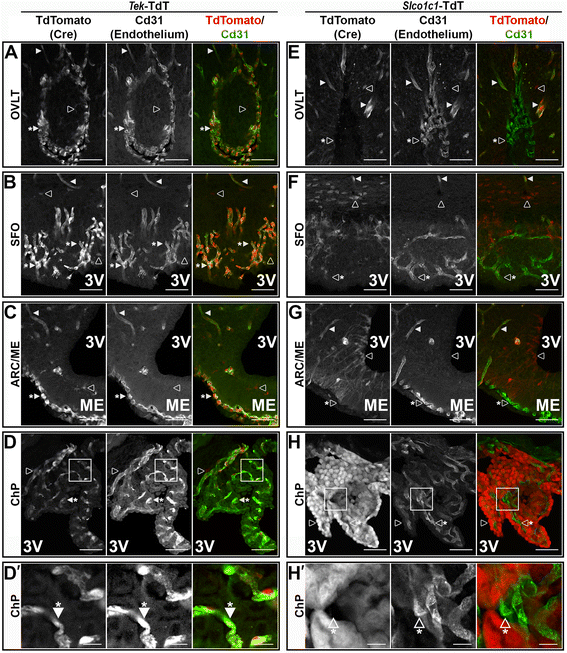
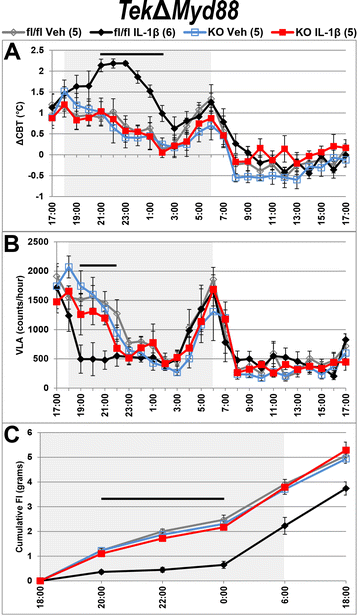
Methods: Intracerebroventricular (ICV) administration of 10 ng of the potent pro-inflammatory cytokine interleukin-1β (IL-1β) was used as an experimental model of central nervous system cytokine production. We examined which cells respond to IL-1β in vivo via fluorescent immunohistochemistry. Using multiple transgenic mouse lines expressing Cre recombinase under the control of cell-specific promoters, we eliminated IL-1β signaling from different populations of cells. Food consumption, body weight, movement, and temperature were recorded in adult male mice and analyzed by two-factor ANOVA to determine where IL-1β signaling is essential for sickness responses.
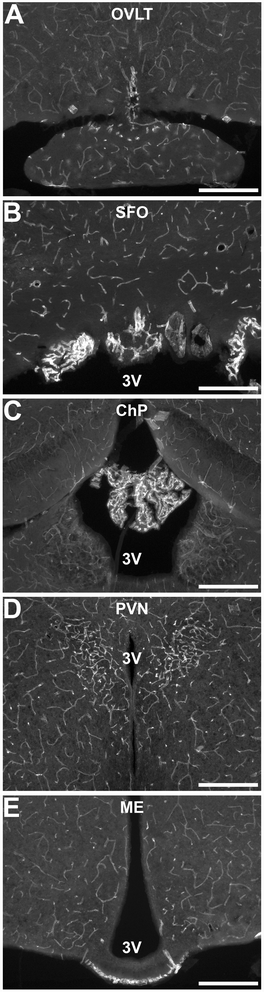
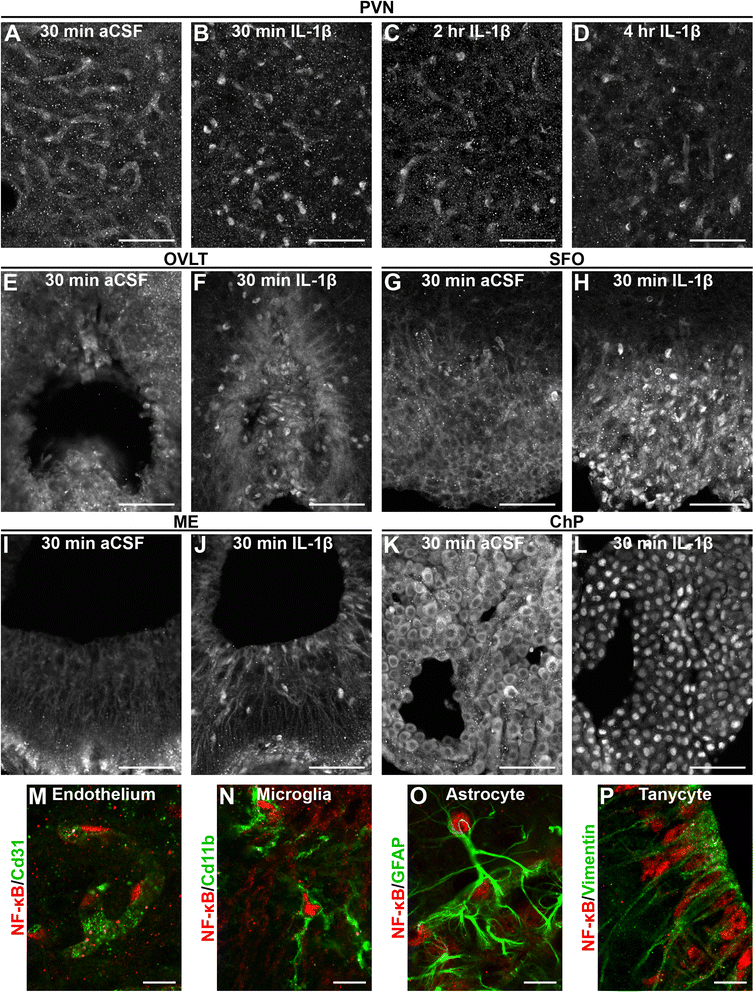
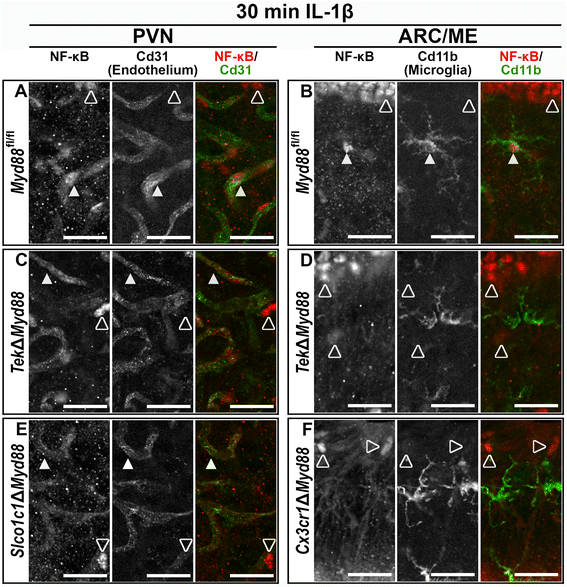
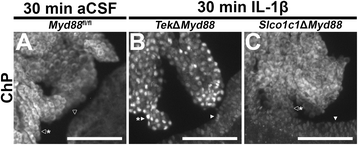
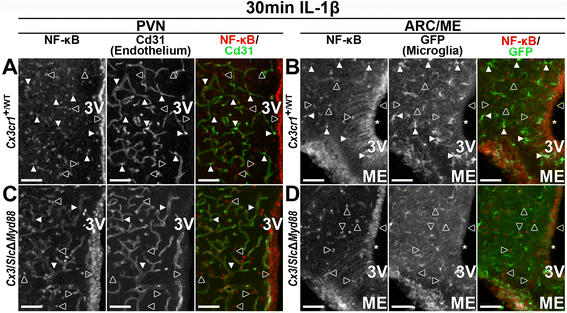
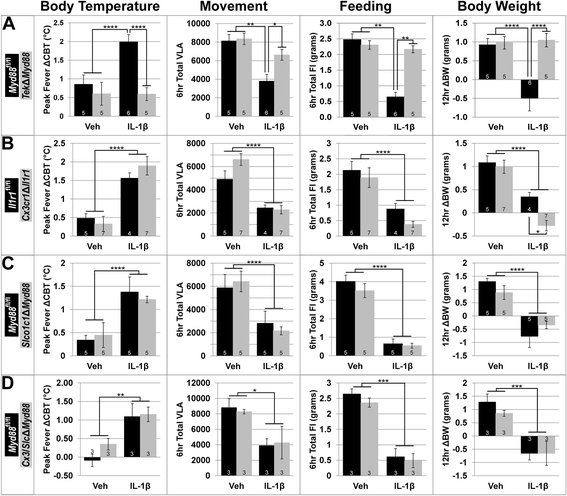
Results: Endothelial cells, microglia, ependymal cells, and astrocytes exhibit nuclear translocation of NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) in response to IL-1β. Interfering with IL-1β signaling in microglia, endothelial cells within the parenchyma of the brain, or both did not affect sickness responses. Only mice that lacked IL-1β signaling in all endothelium including fenestrated capillaries lacked sickness responses.
Conclusions: These experiments show that IL-1β-induced sickness responses depend on intact IL-1β signaling in blood vessels and suggest that fenestrated capillaries act as a critical signaling relay between the immune and nervous systems.
Trial registration: Not applicable.
Keywords: Cytokine; Endothelial cells; Fenestrated capillaries; Hypothalamus; Inflammation; Microglia; Sickness behavior.
Conflict of interest statement
Ethics approval
All animal care, handling, and experimentation were conducted in accordance with the OHSU Institutional Animal Care and Use Committee (IACUC) guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Amplification and propagation of interleukin-1β signaling by murine brain endothelial and glial cells.J Neuroinflammation. 2017 Jul 1;14(1):133. doi: 10.1186/s12974-017-0908-4. J Neuroinflammation. 2017. PMID: 28668091 Free PMC article.
-
Expression of myeloid differentiation factor 88 in neurons is not requisite for the induction of sickness behavior by interleukin-1β.J Neuroinflammation. 2012 Oct 3;9:229. doi: 10.1186/1742-2094-9-229. J Neuroinflammation. 2012. PMID: 23031643 Free PMC article.
-
Differences in the relative involvement of peripherally released interleukin (IL)-6, brain IL-1β and prostanoids in mediating lipopolysaccharide-induced fever and sickness behavior.Psychoneuroendocrinology. 2011 Jun;36(5):608-22. doi: 10.1016/j.psyneuen.2010.09.003. Epub 2010 Oct 5. Psychoneuroendocrinology. 2011. PMID: 20926198
-
The Role of Pro-Inflammatory and Regulatory Signaling by IL-33 in the Brain and Liver: A Focused Systematic Review of Mouse and Human Data and Risk of Bias Assessment of the Literature.Int J Mol Sci. 2020 May 30;21(11):3933. doi: 10.3390/ijms21113933. Int J Mol Sci. 2020. PMID: 32486265 Free PMC article.
-
Immune-to-Brain Communication Pathways in Inflammation-Associated Sickness and Depression.Curr Top Behav Neurosci. 2017;31:73-94. doi: 10.1007/7854_2016_37. Curr Top Behav Neurosci. 2017. PMID: 27677781 Review.
Cited by
-
Hordeum vulgare ethanolic extract mitigates high salt-induced cerebellum damage via attenuation of oxidative stress, neuroinflammation, and neurochemical alterations in hypertensive rats.Metab Brain Dis. 2023 Oct;38(7):2427-2442. doi: 10.1007/s11011-023-01277-5. Epub 2023 Aug 30. Metab Brain Dis. 2023. PMID: 37646962 Free PMC article.
-
Cell-specific conditional deletion of interleukin-1 (IL-1) ligands and its receptors: a new toolbox to study the role of IL-1 in health and disease.J Mol Med (Berl). 2020 Jul;98(7):923-930. doi: 10.1007/s00109-020-01928-5. Epub 2020 May 29. J Mol Med (Berl). 2020. PMID: 32468079 Free PMC article. Review.
-
TRIF is a key inflammatory mediator of acute sickness behavior and cancer cachexia.Brain Behav Immun. 2018 Oct;73:364-374. doi: 10.1016/j.bbi.2018.05.021. Epub 2018 May 28. Brain Behav Immun. 2018. PMID: 29852290 Free PMC article.
-
Peripheral-to-central immune communication at the area postrema glial-barrier following bleomycin-induced sterile lung injury in adult rats.Brain Behav Immun. 2020 Jul;87:610-633. doi: 10.1016/j.bbi.2020.02.006. Epub 2020 Feb 22. Brain Behav Immun. 2020. PMID: 32097765 Free PMC article.
-
Localization of brain neuronal IL-1R1 reveals specific neural circuitries responsive to immune signaling.J Neuroinflammation. 2024 Nov 19;21(1):303. doi: 10.1186/s12974-024-03287-1. J Neuroinflammation. 2024. PMID: 39563437 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

